Two-color fluorescence PCR primer, probe and method for quickly distinguishing canine parvovirus vaccine strain from wild strain
A canine parvovirus, two-color fluorescence technology, applied in the field of identification of virus vaccine virus and wild virus strain, can solve the problems of limited application, time-consuming, complicated operation, etc., and achieves the effects of good repeatability, reduced time, and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Example 1 Primers and Probes
[0089] After screening a large number of designed primers and probes, it was found that primer pairs F1 / R1, F2 / R2, and probes P1 and P2 had the best effect on distinguishing canine parvovirus vaccine strains from wild strains by two-color fluorescence method. The base sequence is shown below.
[0090] F1: 5'-TATAGCACATCAAGATACAGGAAGA-3' (SEQ ID NO: 1),
[0091] R1: 5'-TCCAATTGGATCTGTTGG-3' (SEQ ID NO: 2),
[0092] Probe P1: 5'-FAM-CCTATCATCTTCCTGTAACAAATGATAG-BHQ1-3' (SEQ ID NO: 3),
[0093] F2: 5'-TGGAAATCACAGCAAACTC-3' (SEQ ID NO: 4),
[0094] R2: 5'-CTAAAGCCATGTTTCCGT-3' (SEQ ID NO: 5),
[0095] Probe P2: 5'-HEX-CGACCGTAAATAATATGGATAAAACTGCGGTCG-BHQ1-3' (SEQ ID NO: 6).
Embodiment 2
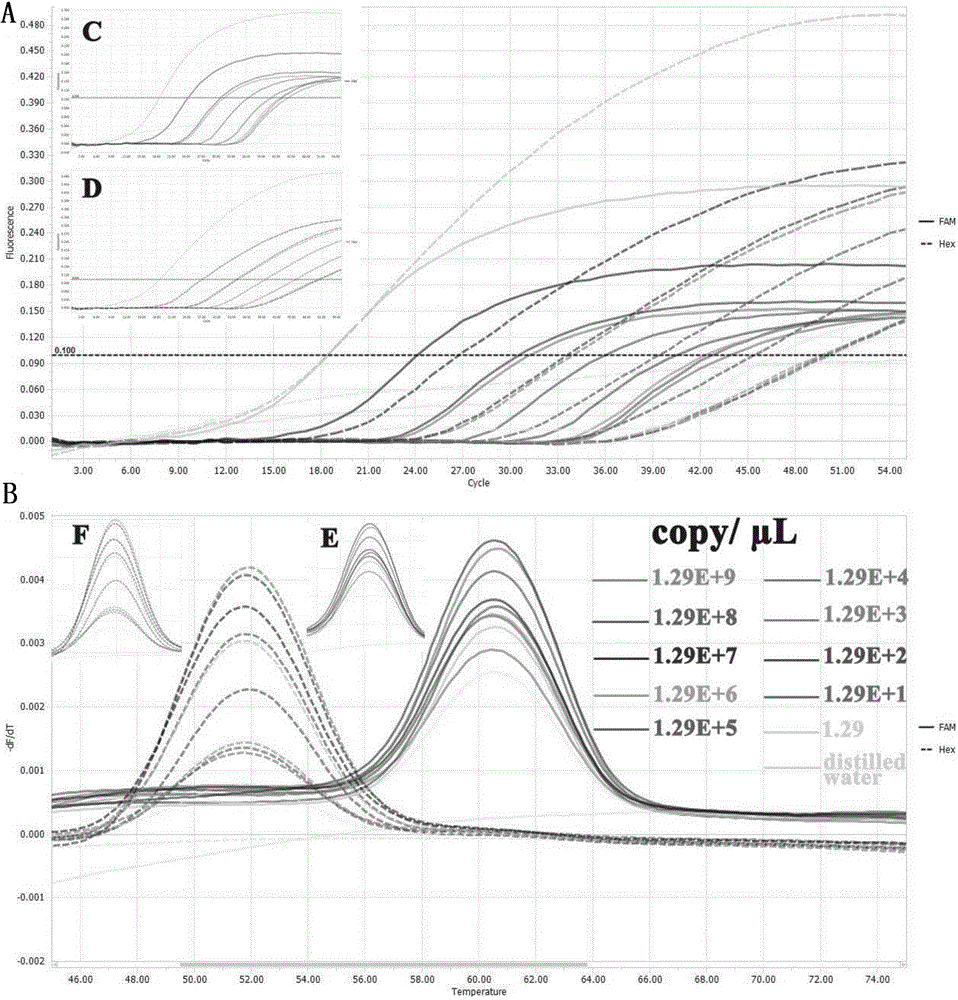
[0096] Example 2 Preparation of standard samples, two-color fluorescent PCR amplification and melting curve analysis
[0097] 1) Extraction of canine parvovirus DNA
[0098] Take samples from diseased materials infected with CPV. The samples of diseased materials can be whole blood, feces, rectal swabs and other samples that are easy to obtain and have no serious harm to the animal body. Take a certain amount and dissolve it in 1mL PBS hydrochloric acid buffer solution, let stand for 10-20min, take 200μl for later use; for CPV vaccine, add 3mL PBS hydrochloric acid buffer solution for dissolution, and take 200ul for later use. Nucleic acid extraction was carried out according to the instructions of TAKARA's MiniBEST Viral RNA / DNA Extraction KitVer.4.0.
[0099] 2) Preparation of standard samples
[0100] In order to verify the feasibility and reliability of the method of the present invention, construct a standard positive sample (correctly sequenced), and provide a positive...
Embodiment 3
[0132] Embodiment 3 specificity experiment
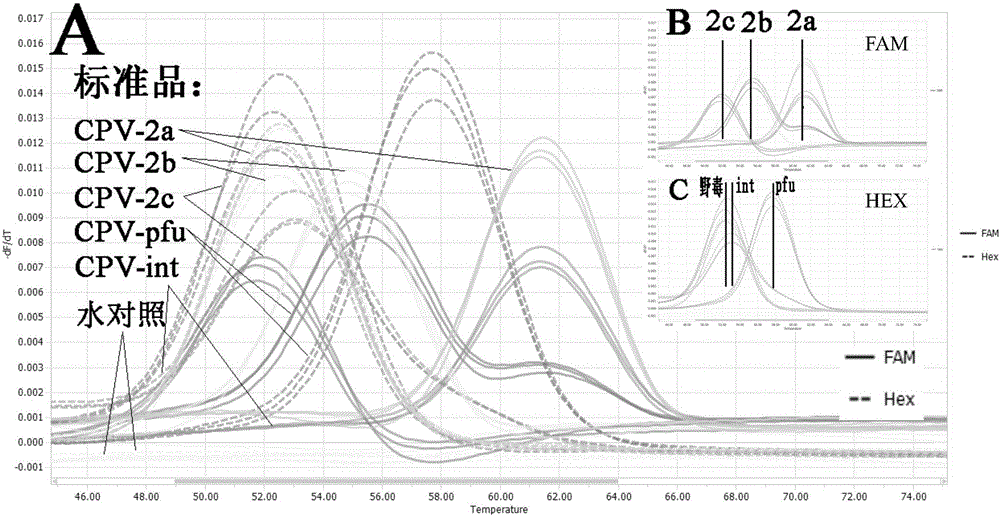
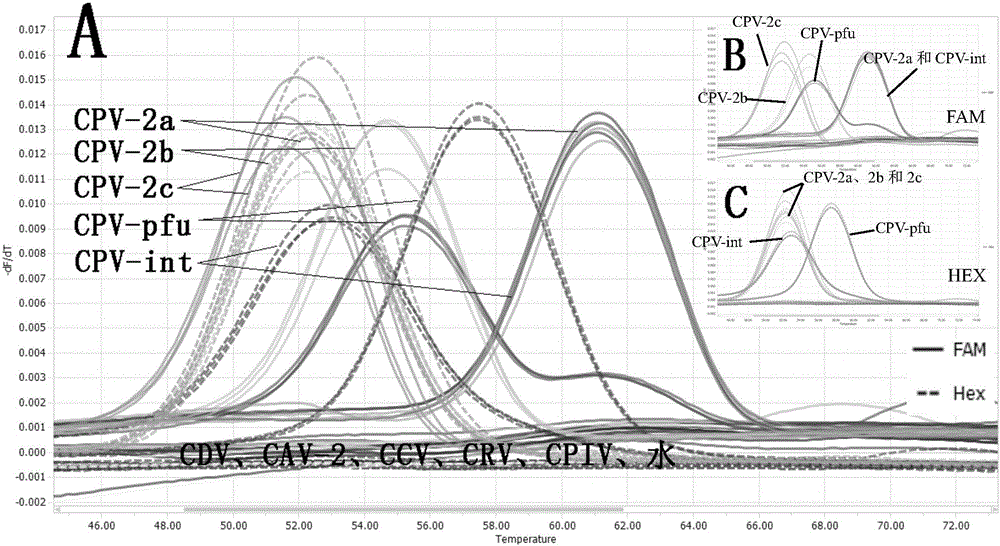
[0133] Next, the detection method established by the present invention is used for specific detection.
[0134] Extract other common canine diarrhea virus nucleic acids, such as canine distemper virμs (CDV), canine adenovirus-2 (Canine adenovirμs type 2, CAV-2), canine coronavirus (Canine coronavirus, CCV), canine rotavirus Viruses (Canine rotavirus, CRV) and canine parainfluenza virus (Canine parainfluenza virus (Canine parainfluenza virμs, CPIV), the RNA of CDV, CCV, CRV and CPIV were reverse-transcribed into cDNA, and the cDNA of the above virus, the nucleic acid of CAV-2 and water were used as The PCR template is analyzed with the PCR amplification reaction and melting curve analysis method in the above-mentioned embodiment 2, and canine parvovirus wild strain CPV-2a, CPV-2b, CPV-2c, vaccine strain CPV-int, CPV- The standard sample of pfu was compared and analyzed, and the peak shape of the melting curve was as follows figure...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com