Synthesis production process of difluorochloroacetic acid
A difluorochloroacetic acid and production process technology, applied in the direction of sulfur compounds, chlorine/hydrogen chloride, acid halide preparation, etc., can solve the problems of many by-products and high prices, achieve high absorption rate and total yield, low energy consumption, Reasonable effect of preparation process design
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
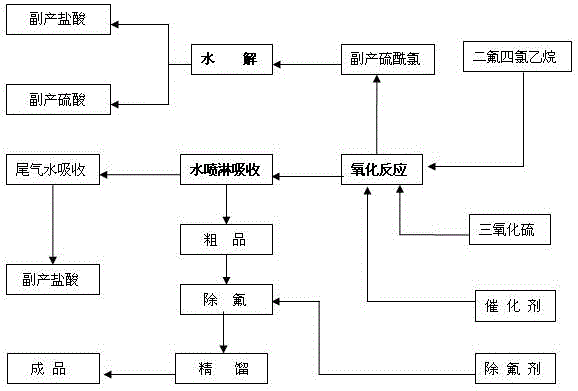
[0023] Such as figure 1 A kind of synthetic production technique of difluorochloroacetic acid shown, comprises the steps:
[0024] (1) Using a batch process, press sulfur trioxide into the oxidation reaction kettle with nitrogen, and start stirring;
[0025] (2) After reaching a certain temperature, add difluorotetrachloroethane and catalyst to the oxidation reactor according to the specified ratio;
[0026] (3) Start the heating reaction and observe the reflux condition. When the temperature of the kettle rises to a certain temperature but the reflux is small, the oxidation reaction of the raw material difluorotetrachloroethane is basically completed;
[0027] (4) Start the operation of steaming by-product sulfuryl chloride, and then carry out the hydrolysis process: adopt a batch process, first add a certain amount of water into the hydrolysis reaction kettle, slowly add sulfuryl chloride dropwise, and stir at a temperature of 20-100°C for hydrolysis reaction, the generate...
Embodiment 2
[0038] A kind of synthetic production technique of difluorochloroacetic acid, comprises the steps:
[0039] (1) Using a batch process, press sulfur trioxide into the oxidation reaction kettle with nitrogen, and start stirring;
[0040] (2) After reaching a certain temperature, add difluorotetrachloroethane and catalyst to the oxidation reactor according to the specified ratio;
[0041] (3) Start the heating reaction and observe the reflux condition. When the temperature of the kettle rises to a certain temperature but the reflux is small, the oxidation reaction of the raw material difluorotetrachloroethane is basically completed;
[0042] (4) Start the operation of steaming by-product sulfuryl chloride, and then carry out the hydrolysis process: adopt a batch process, first add a certain amount of water into the hydrolysis reaction kettle, slowly add sulfuryl chloride dropwise, and stir at a temperature of 20-100°C for hydrolysis reaction, the generated hydrogen chloride gas ...
Embodiment 3
[0053] A kind of synthetic production technique of difluorochloroacetic acid, comprises the steps:
[0054] (1) Using a batch process, press sulfur trioxide into the oxidation reaction kettle with nitrogen, and start stirring;
[0055] (2) After reaching a certain temperature, add difluorotetrachloroethane and catalyst to the oxidation reactor according to the specified ratio;
[0056] (3) Start the heating reaction and observe the reflux condition. When the temperature of the kettle rises to a certain temperature but the reflux is small, the oxidation reaction of the raw material difluorotetrachloroethane is basically completed;
[0057] (4) Start the operation of steaming by-product sulfuryl chloride, and then carry out the hydrolysis process: adopt a batch process, first add a certain amount of water into the hydrolysis reaction kettle, slowly add sulfuryl chloride dropwise, and stir at a temperature of 20-100°C for hydrolysis reaction, the generated hydrogen chloride gas ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com