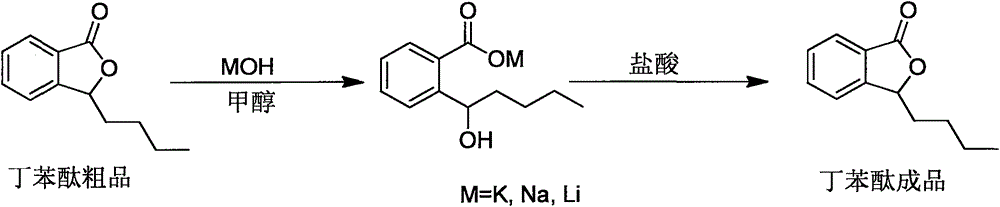
Preparation method of high-purity butylphthalide
A kind of butylphthalide, high-purity technology, applied in the refining field of butylphthalide, can solve the problems of long reaction time, degradation, unfavorable energy consumption and cost control and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1: the preparation of butylphthalide crude product:
[0022] Under nitrogen protection, 90Kg of n-butylmagnesium chloride tetrahydrofuran solution was added to the reactor, 10Kg of o-carboxybenzaldehyde was added to 50L of anhydrous tetrahydrofuran to make a solution, and the Grignard reagent was added dropwise, and the temperature was controlled not to exceed 55 °C. First add 100L of saturated ammonium chloride to quench the reaction, then add 50L of HCl (4M) solution to adjust the pH of the system to about 2.0, continue to stir and let stand for stratification, the organic phase is depressurized to remove the solvent, add 50L of dichloromethane to dilute, 50L of saturated carbonic acid The solution was washed with sodium hydrogen solution, left to stand for layers, and the organic phase was concentrated under reduced pressure to obtain 12.3Kg of a crude product of racemic-3-n-butylphthalide.
Embodiment 2
[0023] Example 2: Refinement of crude butylphthalide (potassium salt):
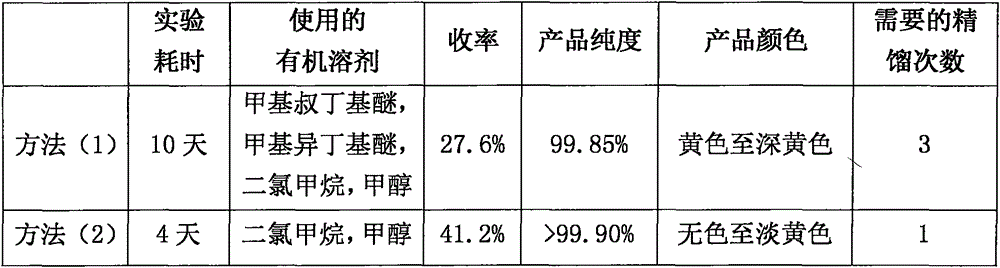
[0024] A. 1.23Kg of racemic-3-n-butylphthalide crude product was added to the reaction flask connected with the rectification separation device, and the pressure was reduced to a vacuum degree of 1-2mbar; Then the temperature was raised to 220° C., and the fractions at 130-140° C. were collected to obtain 0.99 kg of racemic-3-n-butylphthalide fraction; the HPLC purity was 99.4%.
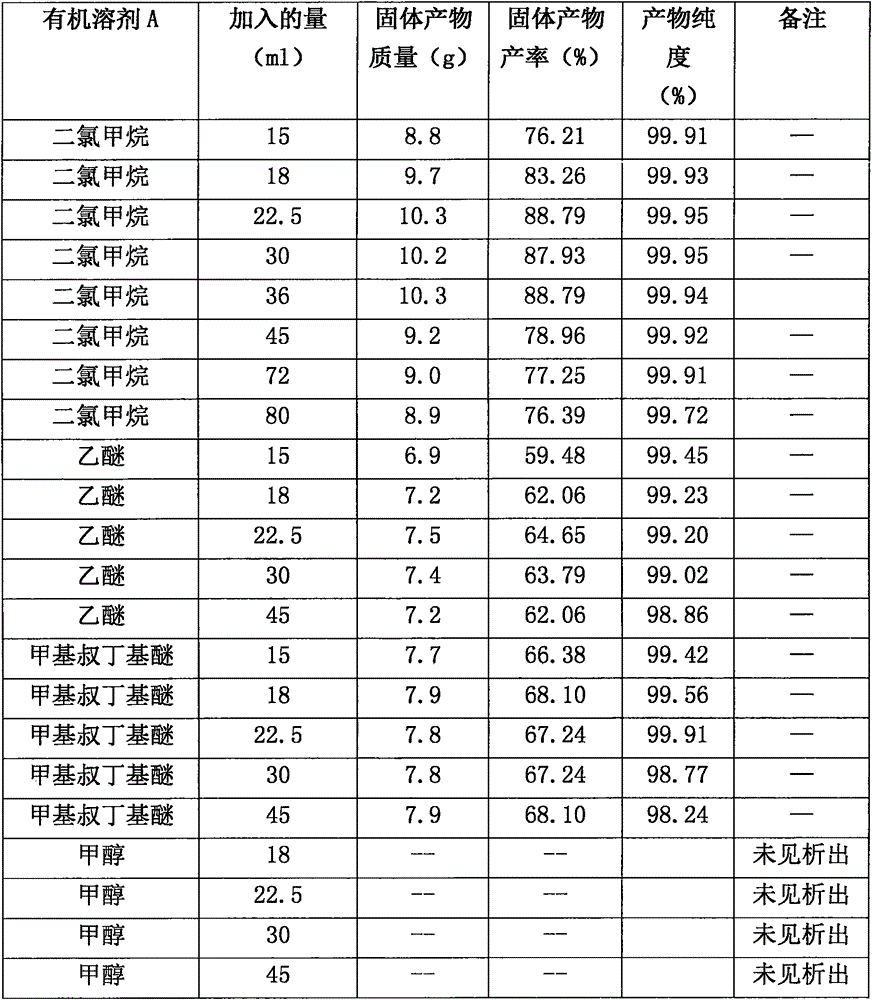
[0025] B. Add 0.99Kg of racemic-3-n-butylphthalide fraction and 2.2L of methanol into a single-necked flask, stir to dissolve, add a solution prepared in advance of 0.31Kg of potassium hydroxide and 1.33L of methanol, heat to 65°C and react for 1h, Concentrate to remove methanol, add 25L of dichloromethane, stir to precipitate a large amount of solid, filter, and dry to obtain 1.19Kg of solid.
[0026] C. 1.19Kg 1-hydroxypentyl-2-potassium benzoate and 2.4L water were added to the reaction flask, stirred and dissolved, then the...
Embodiment 3
[0027] Example 3: Synthesis of sodium 1-hydroxypentyl-2-benzoate
[0028] Add 9 g of racemic-3-n-butylphthalide fraction and 25 mL of methanol into a single-neck flask, stir to dissolve, add sodium hydroxide methanol solution (3.3 g / 12 mL), heat to 65 °C for 1 h, heat and evaporate to dryness to obtain a white solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com