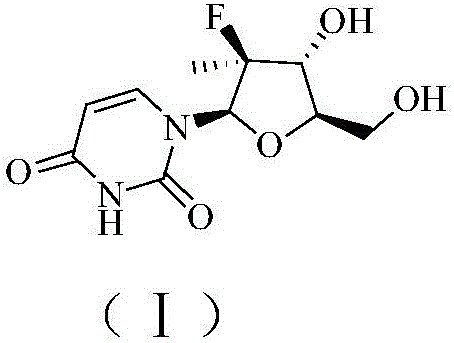
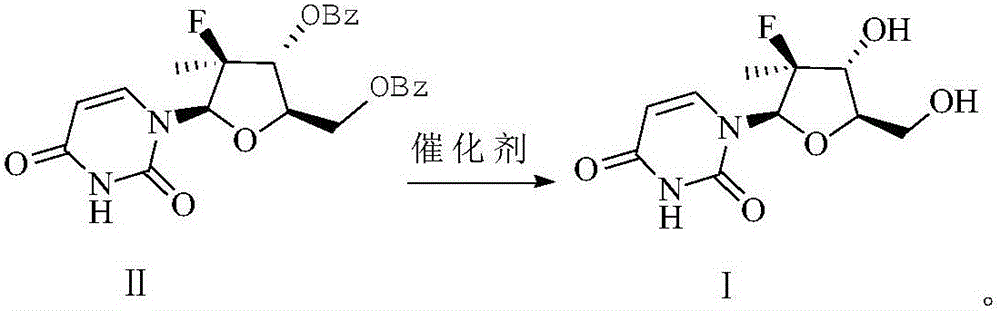
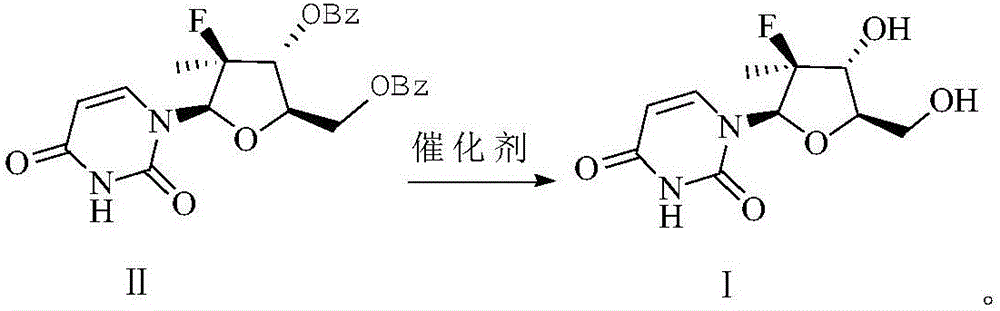
Preparation method of (2'R)-2'-deoxy-2'-fluoro-2'-methyluridine
A technology of methyl uridine and methyl uridine is applied in the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., and can solve the problems of increasing the difficulty of purifying formula I, increasing production costs, and expensive organic bases. , to achieve the effect of simplifying the post-processing method, reducing the production cost and shortening the reaction time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Add 140.00g (0.30mol) of uridine compound, 700mL of anhydrous methanol, 1.00g (0.03mol) of sodium hydroxide and 1.00g (0.01mol) of anhydrous sodium sulfate into a dry and clean 1L three-necked flask, stir, and the reaction solution is It was white and turbid, and the temperature was raised to reflux. When the reaction system was brown and clear, it was reacted for 7 hours. The reaction solution was concentrated to dryness and recrystallized with ethyl acetate to obtain 82.16 g of crude compound I.
[0018] Add 500 mL of anhydrous methanol to the above crude product and stir until completely dissolved, then add 10% H 2 SO 4 / methanol solution to neutralize the pH of the system, decolorize with activated carbon, concentrate and dry to obtain a white granular solid, which is recrystallized using a mixed solvent of absolute ethanol and ethyl acetate, filtered with suction, and dried to obtain 70.01 g of refined compound Ⅰ, with a yield of 90.02%.
Embodiment 2
[0020] Add 140.00g (0.30mol) of uridine compound, 700mL of anhydrous methanol, 1.00g (0.03mol) of sodium hydroxide and 1.00g (0.01mol) of anhydrous magnesium sulfate into a dry and clean 1L three-necked flask, stir, and the reaction solution is It was white and turbid, and the temperature was raised to reflux. When the reaction system was brown and clear, it was reacted for 7 hours. The reaction solution was concentrated to dryness and recrystallized with ethyl acetate to obtain 81.19 g of crude compound Ⅰ.
[0021] Add 500 mL of anhydrous methanol to the above crude product and stir until completely dissolved, then add 10% H 2 SO 4 / methanol solution to neutralize the pH of the system, decolorize with activated carbon, concentrate and dry to obtain a white granular solid, which is recrystallized using a mixed solvent of absolute ethanol and ethyl acetate, filtered with suction, and dried to obtain 70.11 g of refined compound Ⅰ, with a yield of 90.15%.
Embodiment 3
[0023] Add 140.00g (0.30mol) of uridine compound, 700mL of anhydrous methanol, 1.00g (0.01mol) of sodium carbonate and 1.00g (0.01mol) of anhydrous sodium sulfate into a dry and clean 1L three-necked flask, stir, and the reaction solution turns white It was turbid and heated to reflux. When the reaction system was brown and clear, it was reacted for 8 hours. The reaction solution was concentrated to dryness and recrystallized with ethyl acetate to obtain 78.17 g of crude compound I.
[0024] Add 500 mL of anhydrous methanol to the above crude product and stir until completely dissolved, then add 10% H 2 SO 4 / methanol solution to make the pH of the system to neutral, decolorize with activated carbon, concentrate and dry to obtain a white granular solid, which is recrystallized using a mixed solvent of absolute ethanol and ethyl acetate, filtered with suction, and dried to obtain 68.12 g of refined compound Ⅰ, with a yield of 87.59%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com