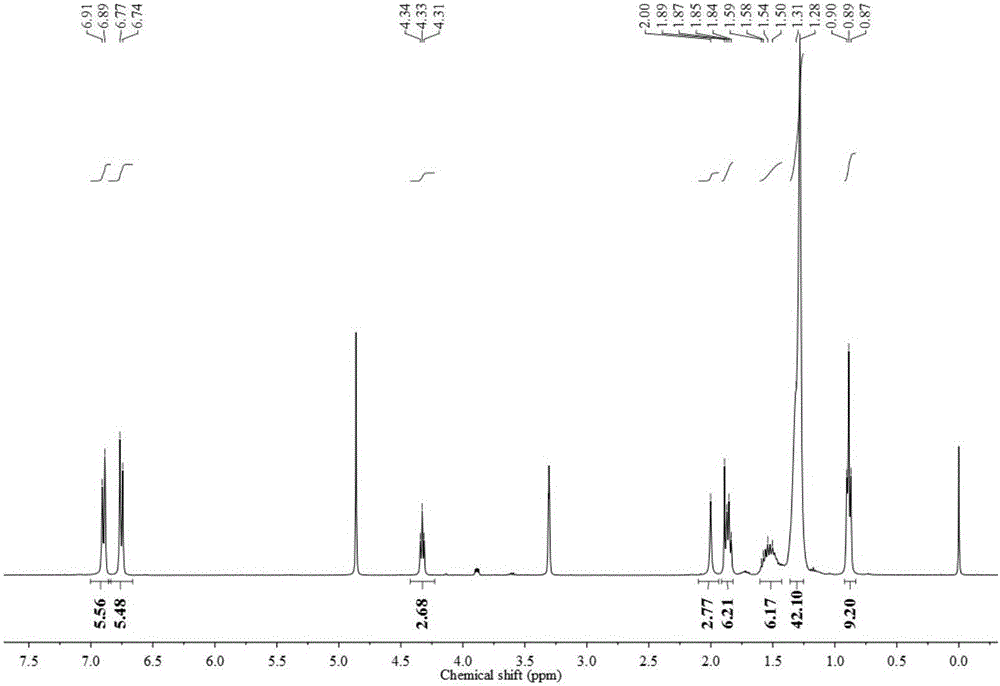
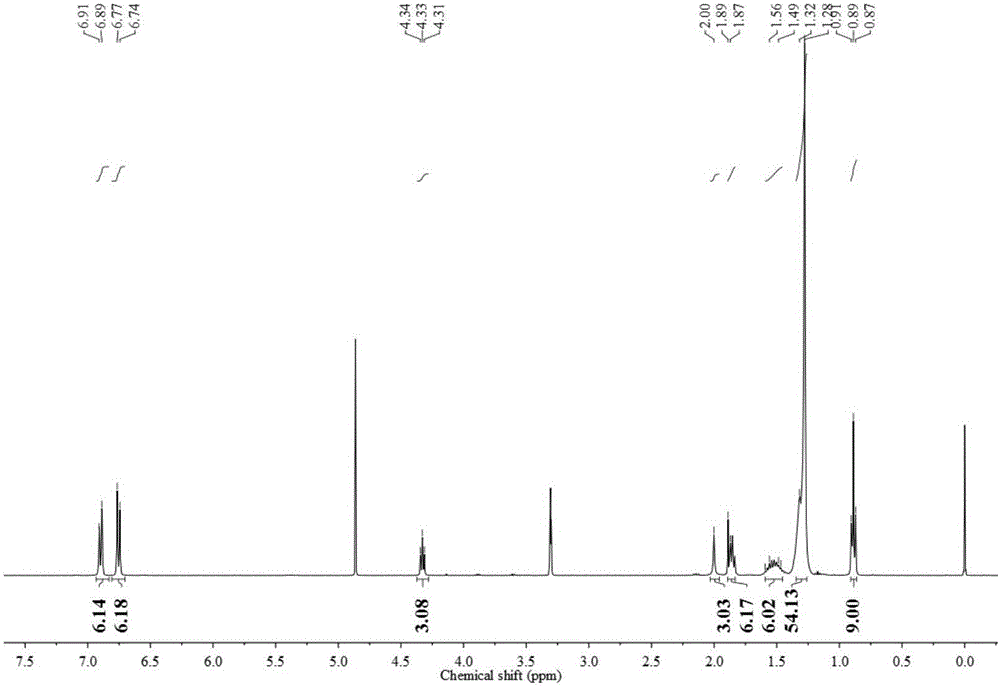
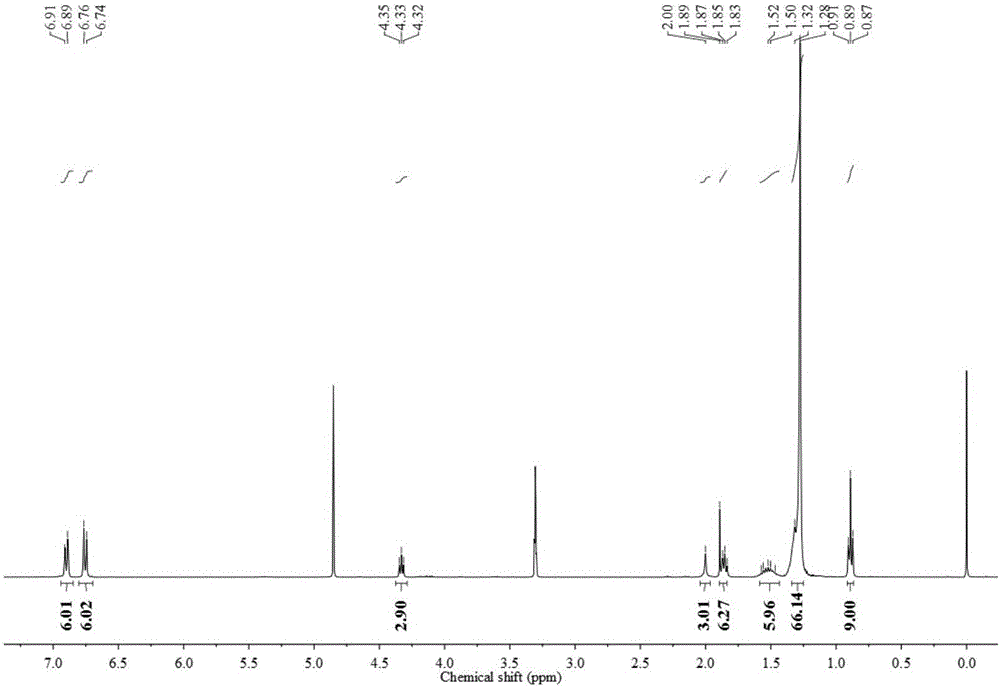
Trimeric anionic surfactant with long rigid linking group and viscoelastic solution formed by trimeric anionic surfactant
A surfactant and rigid connection technology, applied in the field of viscoelastic solution, trimeric anionic surfactant and viscoelastic solution formed by it, can solve system pollution, reduce application performance, high application cost of surfactant viscoelastic solution To reduce the cost of use and eliminate the effect of chromatographic separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1: the synthesis of α-bromododecanoic acid methyl ester. Add dodecanoic acid (68.8g, 0.4mol) into a 500mL three-neck flask, melt at 65°C, slowly add thionyl chloride (67.6g, 0.56mol) dropwise under stirring, and raise the temperature to 90°C after the dropwise addition, Add a little elemental iodine as an initiator, slowly add liquid bromine (73.6g, 0.46mol) dried with concentrated sulfuric acid dropwise, stir and reflux for 12 hours, lower the temperature to 65°C, add 60mL of anhydrous methanol dropwise under stirring, and complete the addition Then reflux for 2-4 hours. After cooling, wash with water 3-4 times, then wash the organic layer with 50 mL of saturated sodium sulfite solution until neutral, and finally wash with water 3-4 times. The organic layer was dried overnight by adding anhydrous magnesium sulfate. After filtration, the crude product was distilled under reduced pressure to obtain α-bromododecanoic acid methyl ester. 154~158℃ / 5mmHg.
Embodiment 2
[0020] Embodiment 2: the synthesis of α-bromotetradecanoic acid methyl ester. Add tetradecanoic acid (150g, 0.6568mol) into a 500mL three-necked flask, melt at 65°C, slowly add thionyl chloride (106.6g, 0.821mol) dropwise under stirring, raise the temperature to 90°C after the dropwise addition, add A little iodine as an initiator, slowly add liquid bromine (131.2g, 0.821mol) dried by concentrated sulfuric acid dropwise, stir and reflux for 12 hours, lower the temperature to 65°C, add 120mL of anhydrous methanol dropwise under stirring, after the addition is complete Reflux for 2-4 hours. After cooling, wash with water 3-4 times, then wash the organic layer with 50 mL of saturated sodium sulfite solution until neutral, and finally wash with water 3-4 times. The organic layer was dried overnight by adding anhydrous magnesium sulfate. After filtration, the crude product was distilled under reduced pressure to obtain α-bromotetradecanoic acid methyl ester. 175~180℃ / 5mmHg.
Embodiment 3
[0021] Embodiment 3: the synthesis of α-bromohexadecanoic acid methyl ester. Add hexadecanoic acid (150g, 0.58mol) into a 500mL three-neck flask, melt at 65°C, slowly add thionyl chloride (95.1g, 0.73mol) dropwise under stirring, raise the temperature to 90°C after the dropwise addition, add A little elemental iodine was used as an initiator, and liquid bromine (116.6 g, 0.73 mol) dried with concentrated sulfuric acid was slowly added dropwise, stirred and refluxed for 12 hours, and the temperature was lowered to 65°C, and 80 mL of anhydrous methanol was added dropwise with stirring, and after the addition was completed, Reflux for 2-4 hours. After cooling, wash with water 3-4 times, then wash the organic layer with 50 mL of saturated sodium sulfite solution until neutral, and finally wash with water 3-4 times. The organic layer was dried overnight by adding anhydrous magnesium sulfate. After filtration, the crude product was distilled under reduced pressure to obtain α-brom...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com