Formula of density gradient solution used for tissue and cell purification
A technology of density gradient and tissue, which is applied in the field of density gradient solution formulation, can solve the problems such as not being suitable for the islet separation center, achieve ideal tissue and cell separation results, be convenient to use, and promote the effect of cell transplantation research and application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The formula of the density gradient liquid used in a tissue and cell purification process in Example 1 of the present invention is as follows:
[0039] (1) Formula 1: Iodixanol 248.0g, potassium lactobionate 15g, gadopentetate meglumine 5g, RPMI1640 to 1L.
[0040] (2) Preparation process:
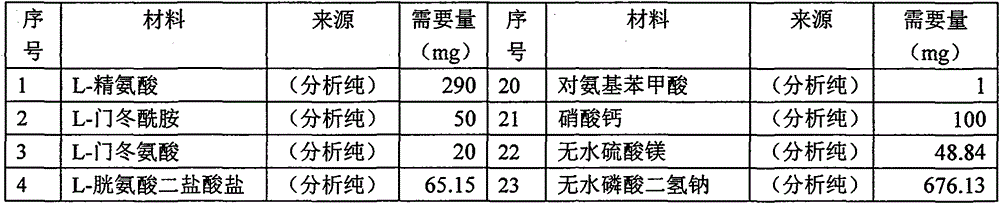
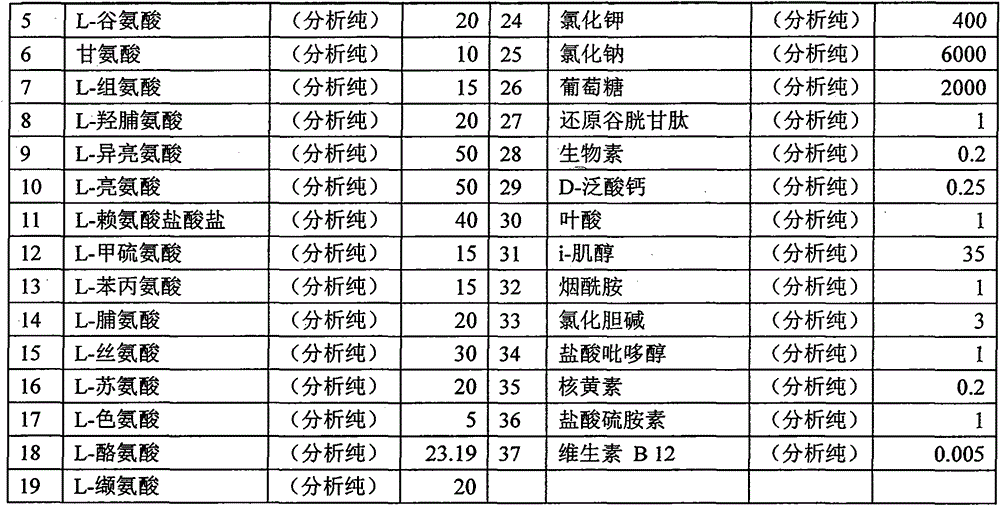
[0041] 1) Take the components of RPMI 1640 (the components of RPMI 1640 can refer to the following table 1), first dissolve with 80% three-distilled water, if necessary, first use HCl to adjust the acid to dissolve, and then use NaOH to adjust the pH value to neutralize, Dilute to 1L with triple distilled water.
[0042] Table 1 RPMI 1640 Component List
[0043]
[0044]
[0045] 2) Accurately weigh 248.0g of iodixanol, 15g of potassium lactobionate, and 5g of gadopentetate meglumine, put them into a 1L sterilized glass bottle, dissolve them in 800ml of the above-mentioned RPMI 1640 solution, and then use the above-mentioned RPMI 1640 solution to dilute to 1L. If necessary...
Embodiment 2
[0064] The formula of high-density density gradient liquid in a tissue and cell purification process of Example 2 of the present invention is as follows:
[0065] (1) Formula: iodixanol 204.0g, diatrizoate sodium 7.36g, gadopentetate meglumine 10.0g, CMRL 1066 to 1L.
[0066] (2) Preparation process:
[0067] 1) Take the components of CMRL 1066 (the components of CMRL 1066 can refer to the following table 2), and first dissolve them with 80% three-distilled water, if necessary, first use HCl to adjust the acid to dissolve, and then use NaOH to adjust the pH value to neutralize, Dilute to 1L with triple distilled water.
[0068] Table 2 CMRL 1066 ingredient list
[0069]
[0070]
[0071] 2) Accurately weigh 204.0g of iodixanol, 7.36g of sodium diatrizoate, and 10.0g of gadopentetate meglumine into a 1L sterilized glass bottle, dissolve with 800ml of the above CMRL 1066 solution, and then use the above CMRL 1066 solution Dilute to 1L. If necessary, use 1N HCl or NaOH ...
Embodiment 3
[0077] A low-density density gradient liquid formula in the tissue and cell purification process of Example 3 of the present invention is as follows:
[0078] (1) Formula: iodixanol 10.22g, diatrizoate sodium 3.59g, gadopentetate meglumine 15g, CMRL 1066 to 250ml.
[0079] (2) Preparation process:
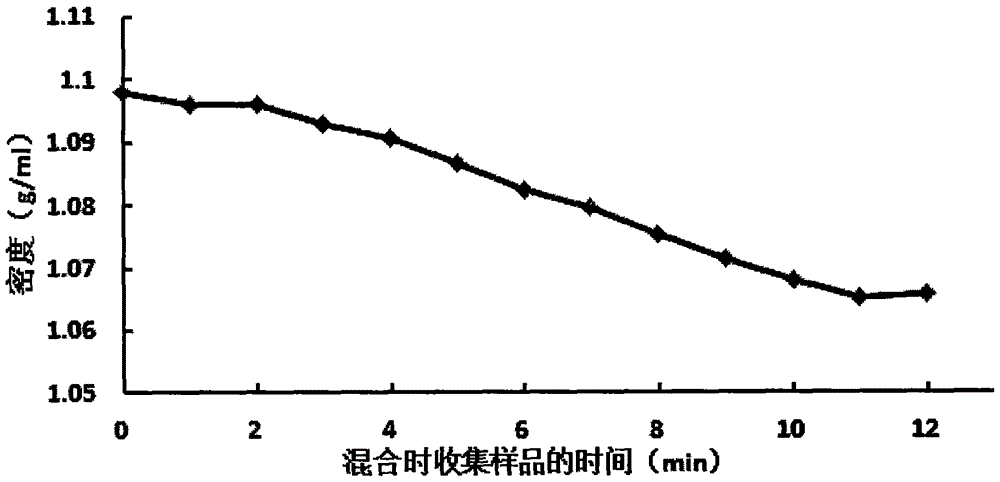
[0080] 1) Accurately weigh 10.22g of iodixanol, 3.59g of sodium diatrizoate, and 15g of gadopentetate meglumine into a 1L sterilized glass bottle, dissolve with 200ml of the above CMRL 1066 solution, and then use the above CMRL 1066 solution to make up to volume to 250ml. If necessary, use 1N HCl or NaOH to adjust the pH to 7.2±0.2, and measure the density with a hand-held electronic density meter to be 1.06±0.01g / ml. If not, add a small amount of iodixanol, sodium diatrizoate or CMRL 1066 to check the density Adjustment.
[0081] 3) In the local A-level clean area under the B-level background, first filter with 0.45 μm microporous membrane and then with 0.22 μm microporous memb...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com