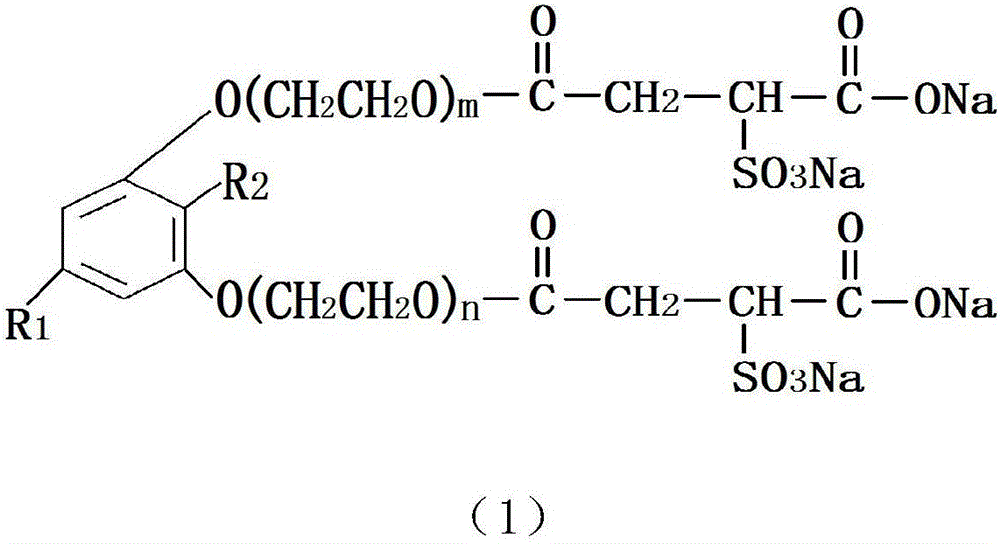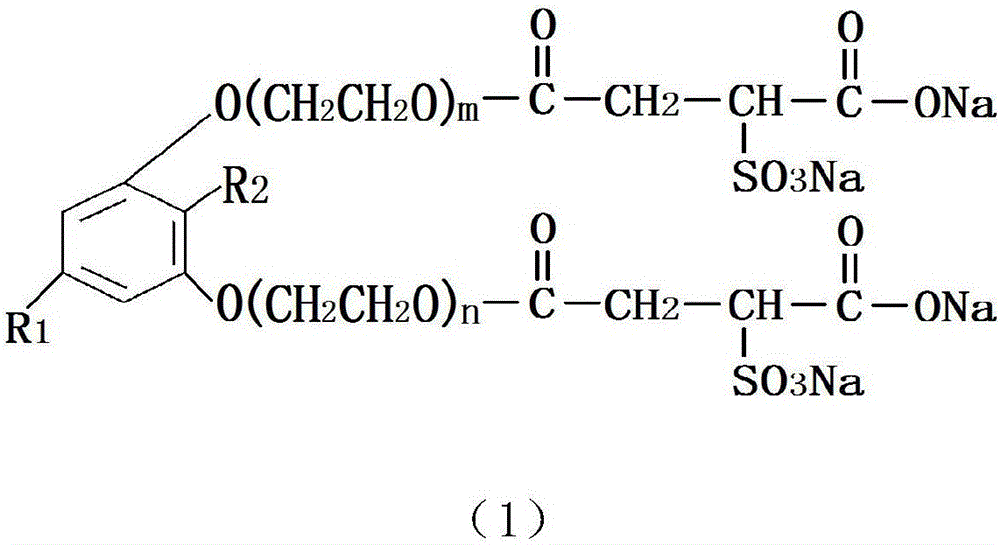Pentadecane (ene) naphthalenediol polyoxyethylene ether sulfo succinate monoester disodium salt and preparation method and application thereof
A technology of diphenol polyoxyethylene ether and sulfosuccinic acid, which can be used in chemical instruments and methods, transportation and packaging, dissolution, etc., and can solve problems such as no surfactants.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Take 316g of refined pentadecane (en)-based resorcinol and put it into the high pressure reactor, add 0.632g of 50% potassium hydroxide solution, perform nitrogen replacement, control the vacuum degree ≥-0.095MPa, and the displacement pressure to 0.15MPa~0.25 MPa; After the nitrogen replacement is completed, the temperature is increased to 110℃~130℃ for dehydration. After the dehydration, the temperature is increased to 135℃~160℃, and 220g of ethylene oxide is added for epoxidation reaction. The temperature of the addition reaction is controlled at 130℃ ~190℃, the pressure is controlled below 0.3MPa, when the addition reaction is completed, the temperature is lowered to 65℃~75℃, then 0.34g of acetic acid is added to neutralize the pH to 5.0~8.0, and the pentadecane (ene) m-bis Phenolic polyoxyethylene ether. Put the pentadecyl (en)-based resorcinol (cardiothyl phenol) polyoxyethylene ether into the esterification reactor, pass nitrogen for replacement, and increase the t...
Embodiment 2
[0027] Take 316g of refined pentadecane (en)-based resorcinol and put it into the high pressure reactor, add 0.632g of 50% potassium hydroxide solution, perform nitrogen replacement, control the vacuum degree ≥-0.095MPa, and the displacement pressure to 0.15MPa~0.25 MPa; After the nitrogen replacement is completed, the temperature is increased to 110℃~130℃ for dehydration. After the dehydration is completed, the temperature is increased to 135℃~160℃, and 264g of ethylene oxide is added for epoxidation reaction. The temperature of the addition reaction is controlled at 130℃ ~190℃, the pressure is controlled below 0.3MPa, when the addition reaction is completed, the temperature is lowered to 65℃~75℃, then 0.34g of acetic acid is added to neutralize the pH to 5.0~8.0, and the pentadecane (ene) m-bis Phenolic polyoxyethylene ether. Put the pentadecyl (en)-based resorcinol (cardiothyl phenol) polyoxyethylene ether into the esterification reactor, pass nitrogen for replacement, and i...
Embodiment 3
[0029] Take 316g of refined pentadecane (en)-based resorcinol and put it into the high pressure reactor, add 0.632g of 50% potassium hydroxide solution, perform nitrogen replacement, control the vacuum degree ≥-0.095MPa, and the displacement pressure to 0.15MPa~0.25 MPa; After the nitrogen replacement is completed, the temperature is increased to 110℃~130℃ for dehydration. After the dehydration is completed, the temperature is increased to 135℃~160℃, and 352g of ethylene oxide is added for the epoxidation reaction. The temperature of the addition reaction is controlled at 130℃ ~190℃, the pressure is controlled below 0.3MPa, when the addition reaction is completed, the temperature is lowered to 65℃~75℃, then 0.34g of acetic acid is added to neutralize the pH to 5.0~8.0, and the pentadecane (ene) m-bis Phenolic polyoxyethylene ether. Put the pentadecyl (en)-based resorcinol (cardiothyl phenol) polyoxyethylene ether into the esterification reactor, pass nitrogen for replacement, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com