Preparation method for buffering layer material of copper indium gallium selenide film solar battery
A technology of solar cells and copper indium gallium selenide, which is applied in the manufacture of circuits, electrical components, and final products, to achieve the effects of simple equipment, reduced lattice mismatch, and environmentally friendly production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
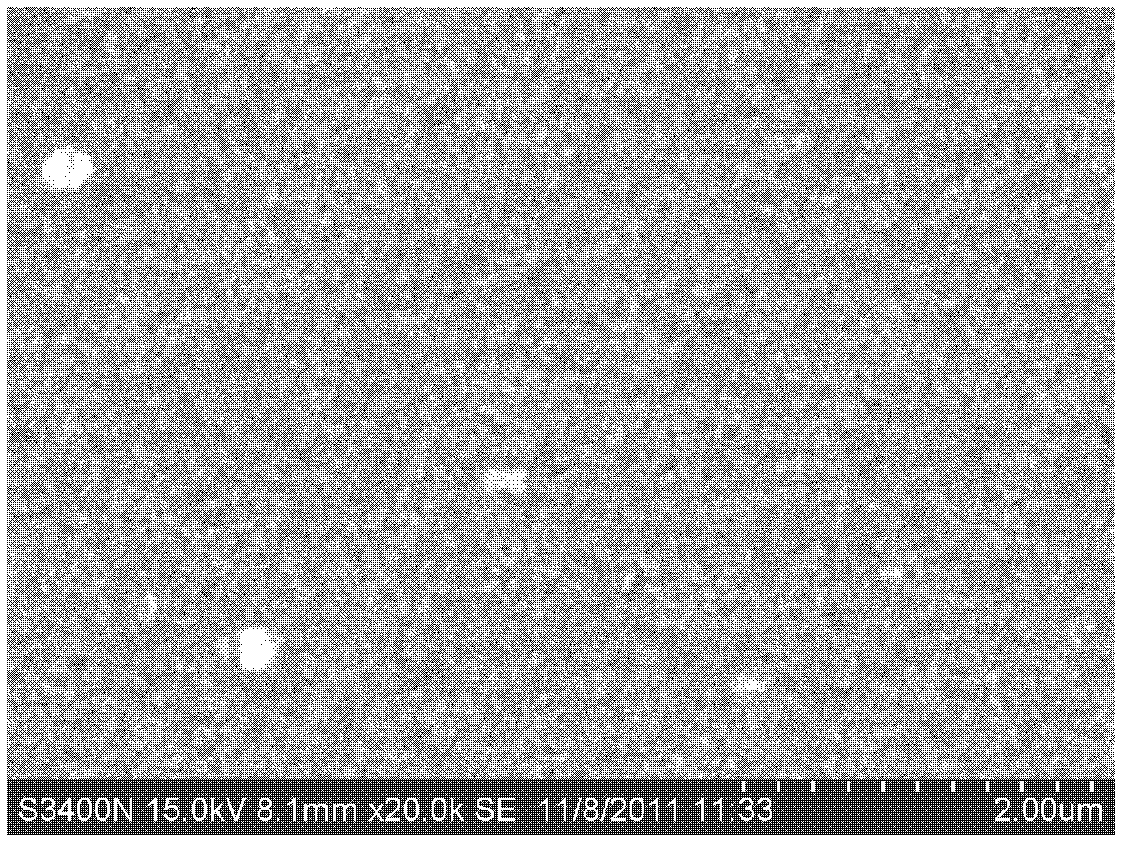
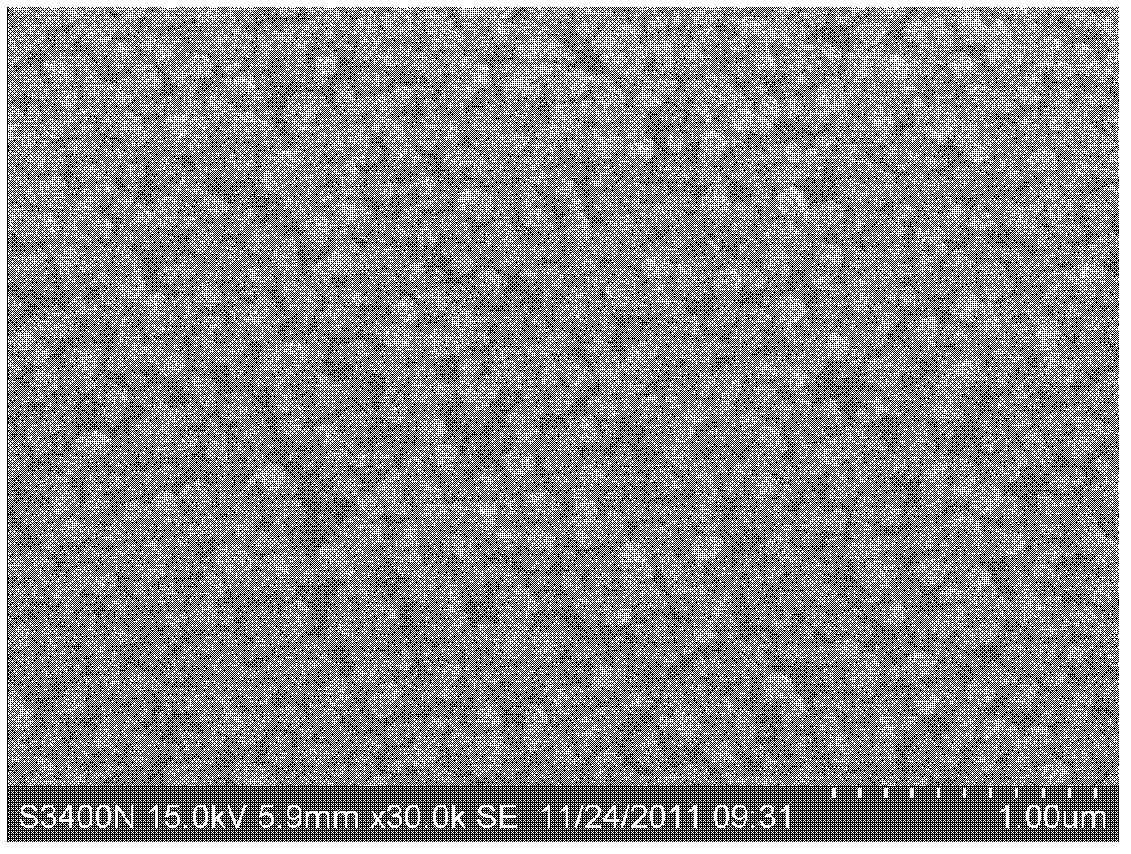
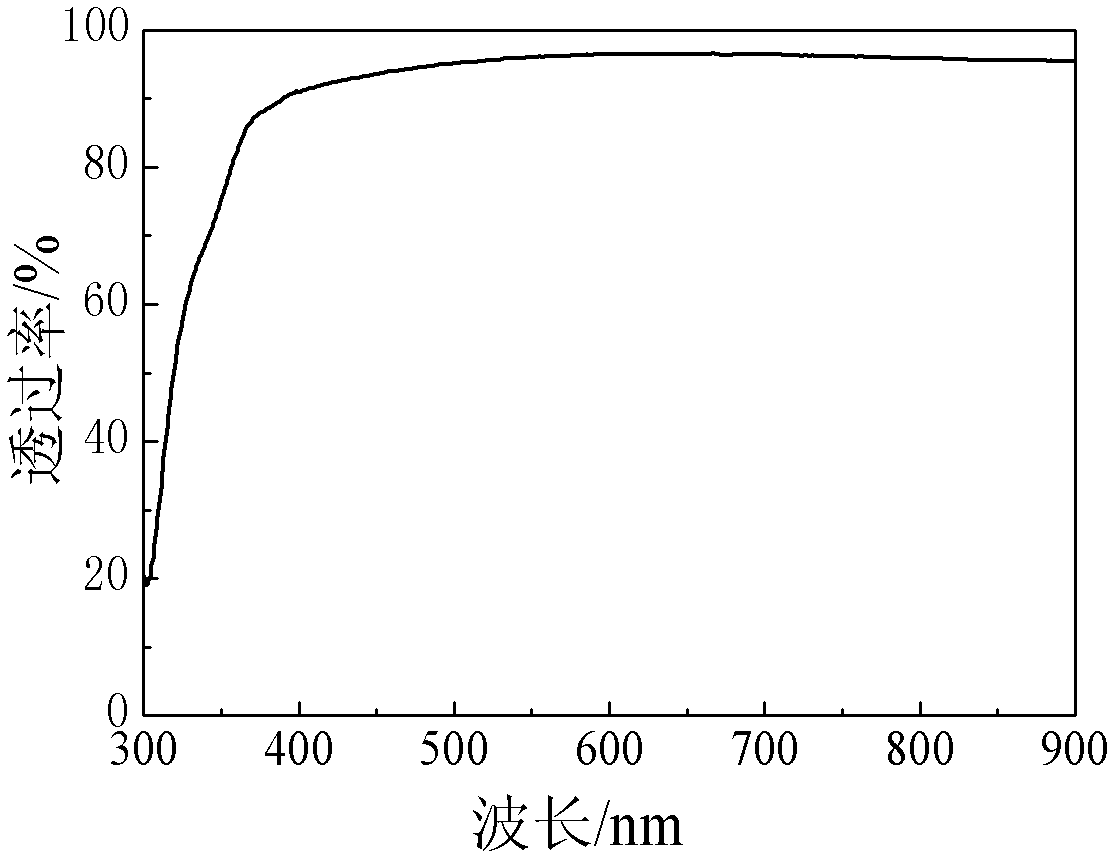
[0038] First measure 0.2mol / L zinc sulfate (ZnSO 4 ·7H 2 O) solution 10ml joins in the clean beaker that volume is 100ml, then adds the sodium citrate (C of 0.1mol / L) while stirring 6 N 5 o 7 Na 3 2H 2 O) solution 3ml, mass fraction is 25% ammoniacal liquor (NH 3 ·H 2 (2) 2ml, then add an appropriate amount of deionized water, so that the total volume of the solution in the beaker is 100ml, finally weighed 0.92g thiourea (SC (NH 2 ) 2 ) powder into the solution and stir well. Put the substrate prepared with the CIGS absorbing layer and the cleaned glass substrate into the solution vertically along the wall of the beaker, and seal the mouth of the beaker with aluminum foil, then put it in 60 o C water bath for constant temperature reaction, the reaction time is 20min. After the reaction, the sample was taken out and rinsed with deionized water to remove colloidal particles and flocculent precipitates adsorbed on the surface of the sample, and then dried to obtain a Zn...
example 2
[0040] First measure 0.2mol / L zinc sulfate (ZnSO 4 ·7H 2 (O) 2ml of the solution is added into a clean beaker with a volume of 100 ml, and then 0.1mol / L of sodium citrate (C 6 N 5 o 7 Na 3 2H 2 O) solution 1ml, mass fraction is 25% ammoniacal liquor (NH 3 ·H 2 (2) 2ml, then add an appropriate amount of deionized water, just make the total volume of the solution added in the beaker be 100ml, finally weigh 0.92g thiourea (SC(NH 2 ) 2 ) powder into the solution. Stir well. Put the substrate prepared with the CIGS absorbing layer and the cleaned glass substrate into the solution vertically along the wall of the beaker, and seal the mouth of the beaker with aluminum foil, and put it in the 70 o C water bath for constant temperature reaction, the reaction time is 60min. After the reaction, the sample was taken out and rinsed with deionized water to remove the colloidal particles and flocculent precipitates adsorbed on the surface of the sample, and then dried to obtain a ...
example 3
[0042] First measure 0.2mol / L zinc sulfate (ZnSO 4 ·7H 2 O) 1ml of the solution is added into a clean beaker with a volume of 100 ml, and then 0.1mol / L of sodium citrate (C 6 N 5 o 7 Na 3 2H 2 O) solution 2ml, mass fraction is 25% ammoniacal liquor (NH 3 ·H 2 (0) 1ml, then add an appropriate amount of deionized water, just make the total volume of the solution added in the beaker be 100ml, and finally weigh 0.46g thiourea (SC(NH 2 ) 2 ) powder into the solution. Stir well. Put the substrate prepared with the CIGS absorbing layer and the cleaned glass substrate into the solution vertically along the wall of the beaker, and seal the mouth of the beaker with aluminum foil, then put it in 50 o Carry out constant temperature reaction in C water bath, the reaction time is 120min. After the reaction, the sample was taken out and rinsed with deionized water to remove the colloidal particles and flocculent precipitates adsorbed on the surface of the sample, and then dried to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com