A high catalytic efficiency beta-glucosidase mutant and its coding gene and application
A glucosidase and catalytic efficiency technology, applied in the field of high catalytic efficiency β-glucosidase mutants and their preparation, can solve the problems of not being completely consistent, different types and degrees, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 Cloning of High Catalytic Efficiency β-Glucosidase Mutant Encoding Gene A-M36E
[0040] The present invention takes the acid β-glucosidase (its amino acid sequence as SEQ ID NO.5) derived from the thermophilic fungus Talaromyces leycettanus JCM12802 as the parent, and uses molecular biology techniques to carry out the acid β-glucosidase sequence Expression after region replacement.
[0041] SEQ ID NO.5 is shown below:
[0042] YGFGGSGWDAAYGRAKAALNKLNQTEKVGIVTGVKWMGGPCVGNTYKPSSIDYPSLCLQDSPLGVRFANPVTAFPAGINAGATWDRSLINARGAAMGAEAKGLGVNVQLGPVAGPLGKNPNSGRIWEGFSNDPYLSGVAMEETIAGMQGSGVQACAKHYIGNEQEHNRETISSNIDDRTLHELYVWPFMNAVKANVASVMCSYNEVNGSWSCENDALLNGLLKTELGFPGYIMSDWNAQHTTVNSANSGLDMTMPGSDFNNPPGSIYWGPNLEAAVANGSVPQSRLDDMVTRILASWYLVGQDEGYPPVAFSSWNGGKANVDVTGDHKSVVRAVARDSIVLLKNDNNALPLRKPKSLAIIGQDATVNPAGPNACSDRGCDTGTLAMGWGSGTAQFPYIVGPLDAIQSQAAADGTNITTSTTDDTTAAASAAASAGTAIVFINSDSGEGYITVEGNAGDRNNLDPWHNGNELVQAVAAVNKNVIVVVHSVGPVILEAILAQPNVKAIVWPGLPGQESGNALVDVLYGSTSPSGKLPYTIAKQFS...
Embodiment 2
[0046] Example 2 Preparation of high catalytic efficiency β-glucosidase mutants with improved pH stability.
[0047] The expression vector pPIC9r was subjected to double enzyme digestion (EcoR I+Not I), and at the same time, the gene A-M36E encoding the high catalytic efficiency β-glucosidase mutant was double enzyme digested (EcoR I+Not I), and the cut code was mature The gene fragment (removing the signal peptide fragment) of the high catalytic efficiency β-glucosidase mutant was connected with the expression vector pPIC9r to obtain the recombinant plasmid pPIC9r- Bgl3A-M1 and pPIC9r-Bgl3A-M2 were transformed into Pichia pastoris GS115 to obtain recombinant yeast strains GS115 / Bgl3A-M1 and GS115 / Bgl3A-M2.
[0048] Take the GS115 strain containing the recombinant plasmid, inoculate it in a 1L Erlenmeyer flask with 300mL of BMGY medium, place it at 30°C, and culture it on a shaker at 220rpm for 48h; then centrifuge the culture solution at 3000g for 5min, discard the supernatan...
Embodiment 3
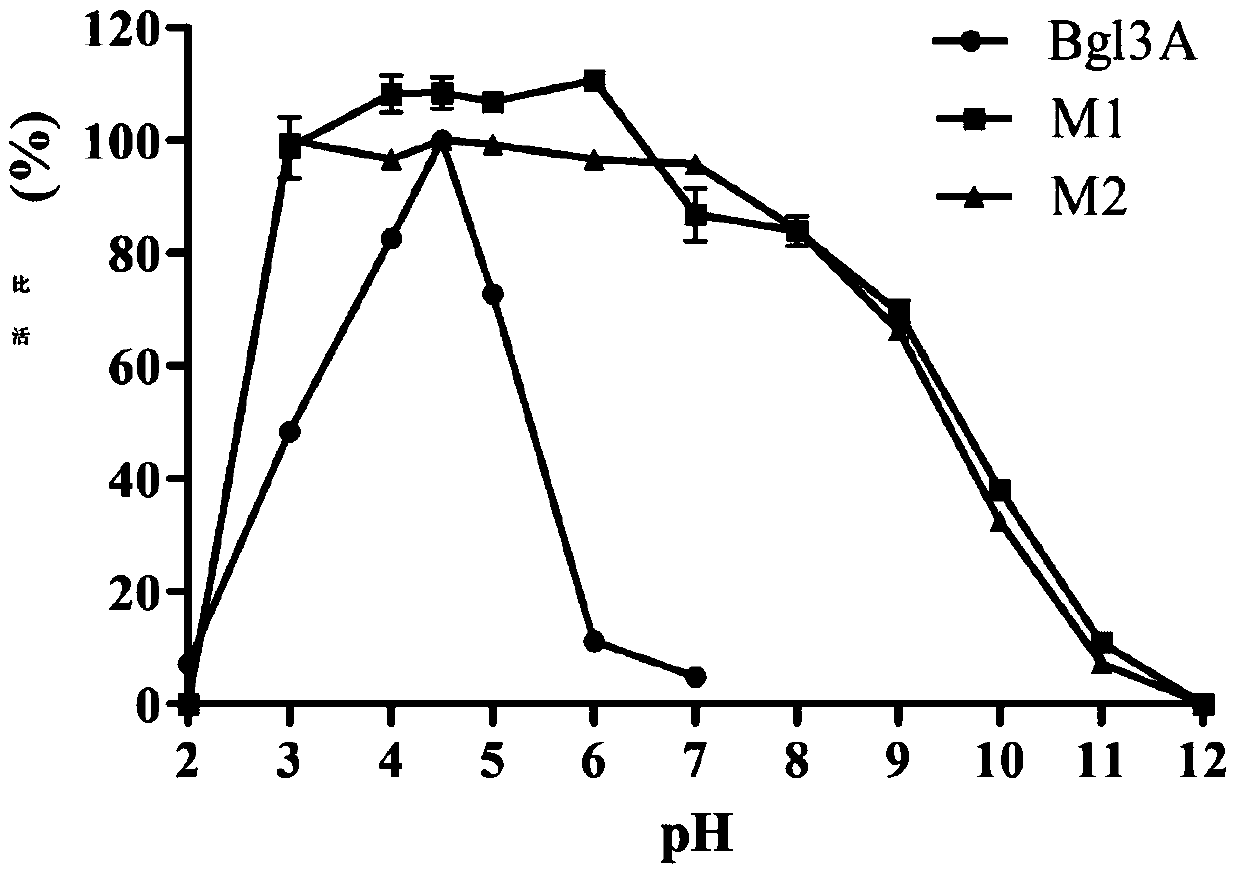
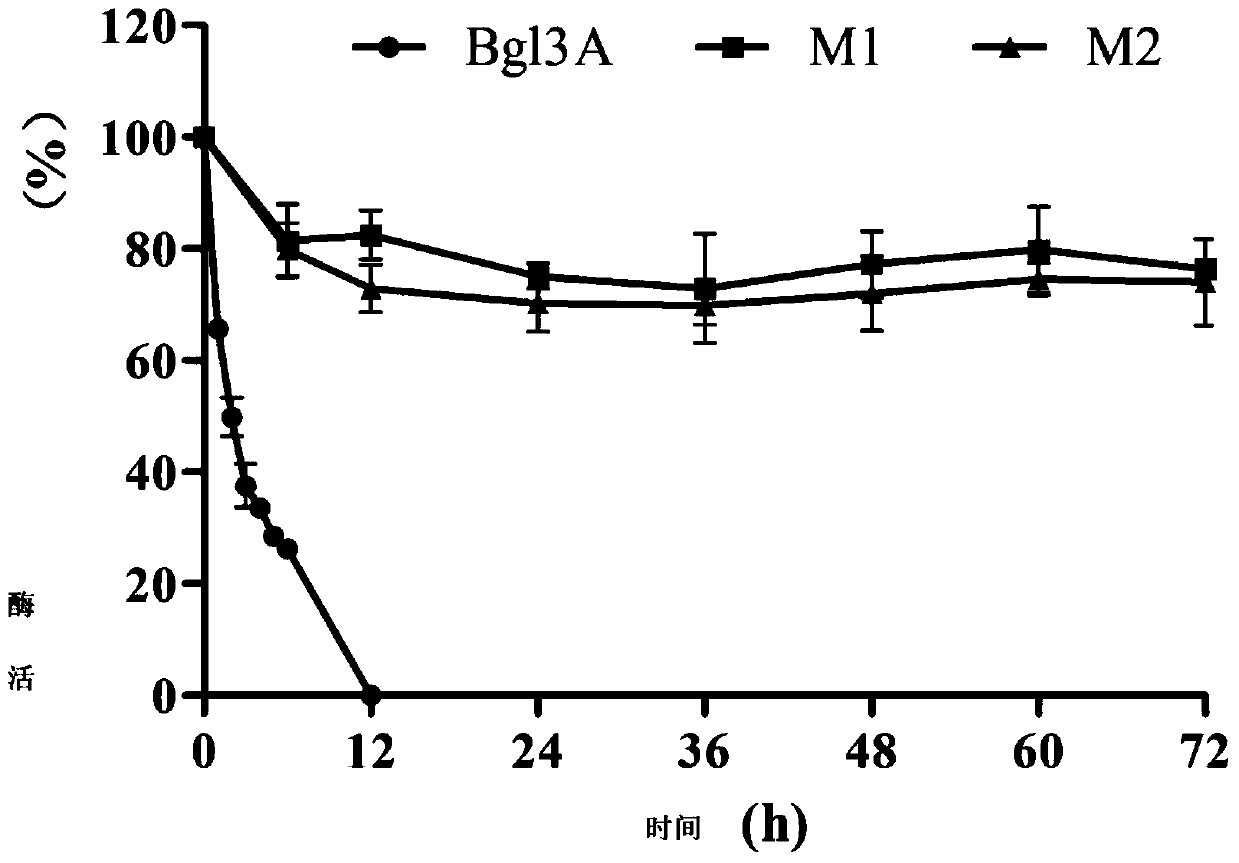
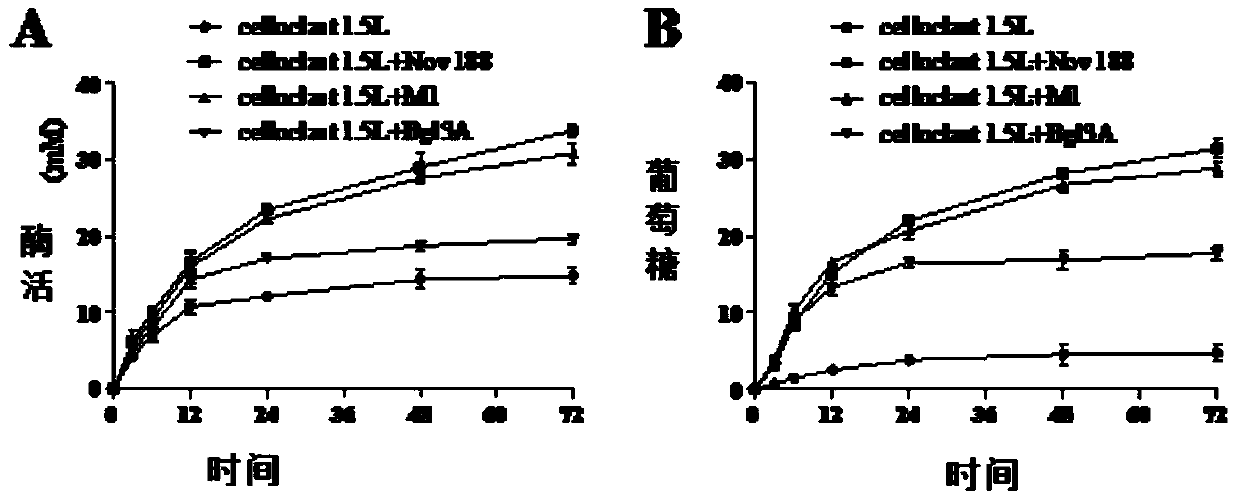
[0049] The activity analysis of the high catalytic efficiency β-glucosidase mutant that embodiment 3 pH stability improves
[0050] Determination of β-glucosidase activity: the amount of the product p-nitrophenol (pNP) generated by enzymatic hydrolysis of the substrate pNPG was measured at 405 nm.
[0051] Reaction steps: mix 125μl 2mM pNPG substrate with 125μl buffer, add 250μl appropriately diluted enzyme solution, react at 70°C for 10min, add 1.5mL 1M Na 2 CO 3 Terminate the reaction and measure the OD using a spectrophotometer 405 value.
[0052] Definition of enzyme activity unit: 1 β-glucosidase activity unit (U) is defined as the amount of enzyme required to decompose the substrate pNPG to generate 1 μmol p-nitrophenol (pNP) per minute under given reaction conditions.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com