Two-photon formaldehyde fluorescent probe and preparation and application thereof
A fluorescent probe and two-photon technology, applied in the field of preparation of new two-photon formaldehyde-based fluorescent probes, can solve few problems, and achieve the effects of strong penetrating ability, reducing fluorescent background, and small cell damage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
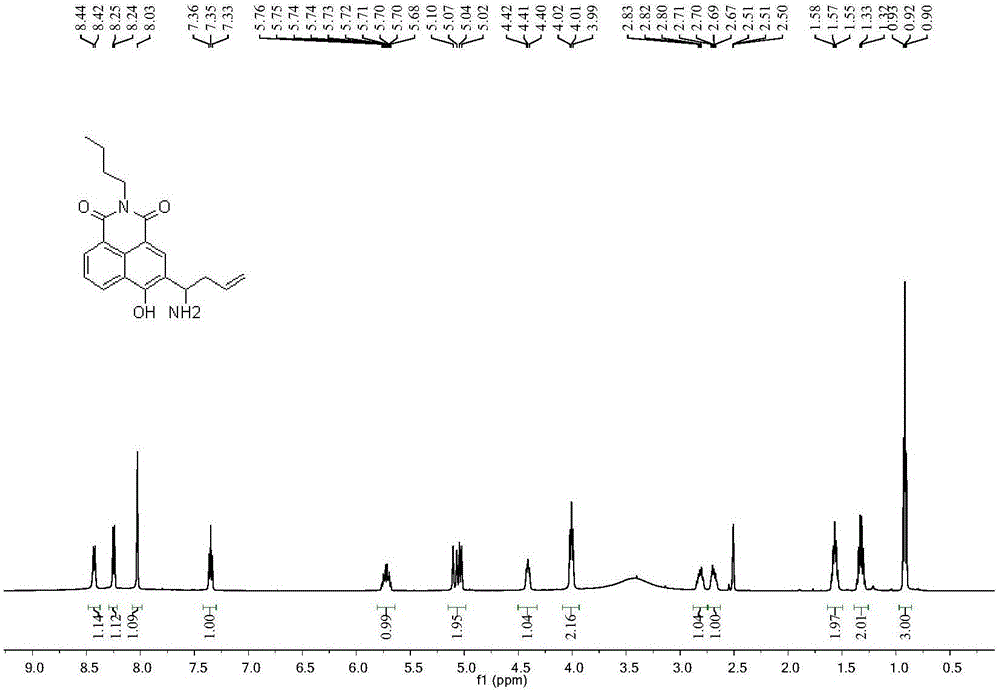
[0034] The synthesis of embodiment 1 probe (I)
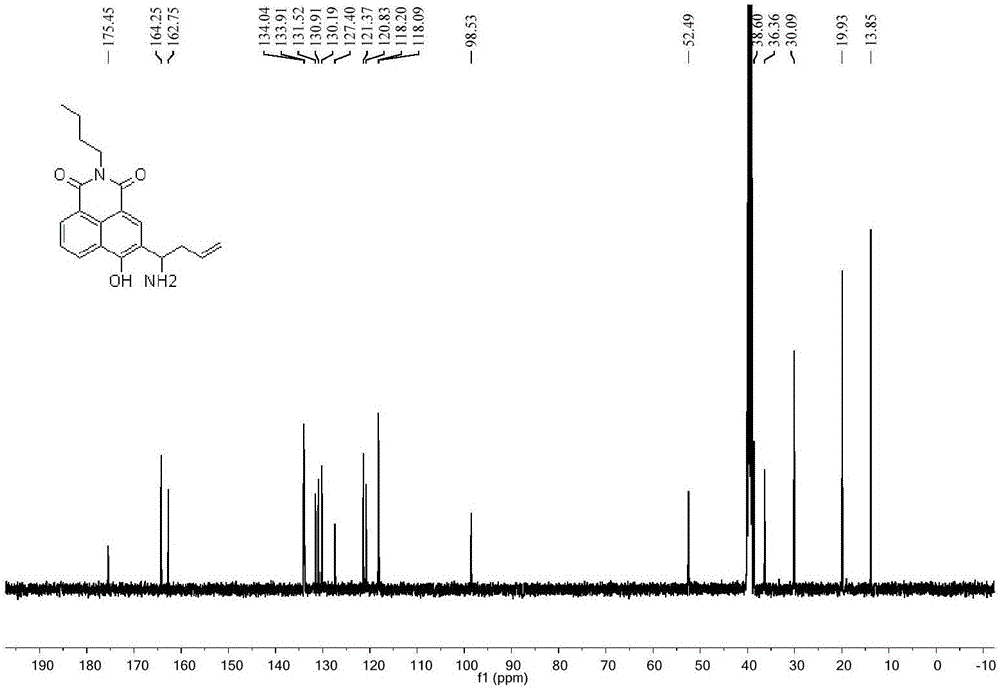
[0035] In a 50mL round bottom flask, add 148mg of compound 4 (0.5mmol) dissolved in 5mL of methanol, ice-bath to 0°C, add 0.72mL ammonia methanol solution (7mol / L 5mmol), react at 0°C for half an hour, then add 168 mg o-di-tert-alcohol propenyl borate (1 mmol). The reaction was brought to room temperature overnight, the mixture was evaporated under reduced pressure to remove the solvent, and the concentrate was separated on a silica gel column (eluted with a mixture of dichloromethane:methanol at a volume ratio of 20:1) to obtain 118 mg of the product with a yield of 70% . H NMR spectrum see figure 1 , see C NMR figure 2 .
[0036] 1 H NMR (500MHz, d 6 -DMSO) δ: 8.43 (d, J = 7.8, 1H), 8.25 (d, J = 7.0, 1H), 8.03 (s, 1H), 7.35 (t, J = 7.6, 1H), 5.72 (ddt, J =13.8,10.1,6.8,1H),5.06(dd,J=25.4,13.6,2H),4.50-4.32(m,1H),4.09-3.94(m,2H),2.88-2.75(m,1H), 2.74-2.63 (m, 1H), 1.64-1.49 (m, 2H), 1.39-1.26 (m, 2H), 0.92 (t, J = 7.4,...
Embodiment 2
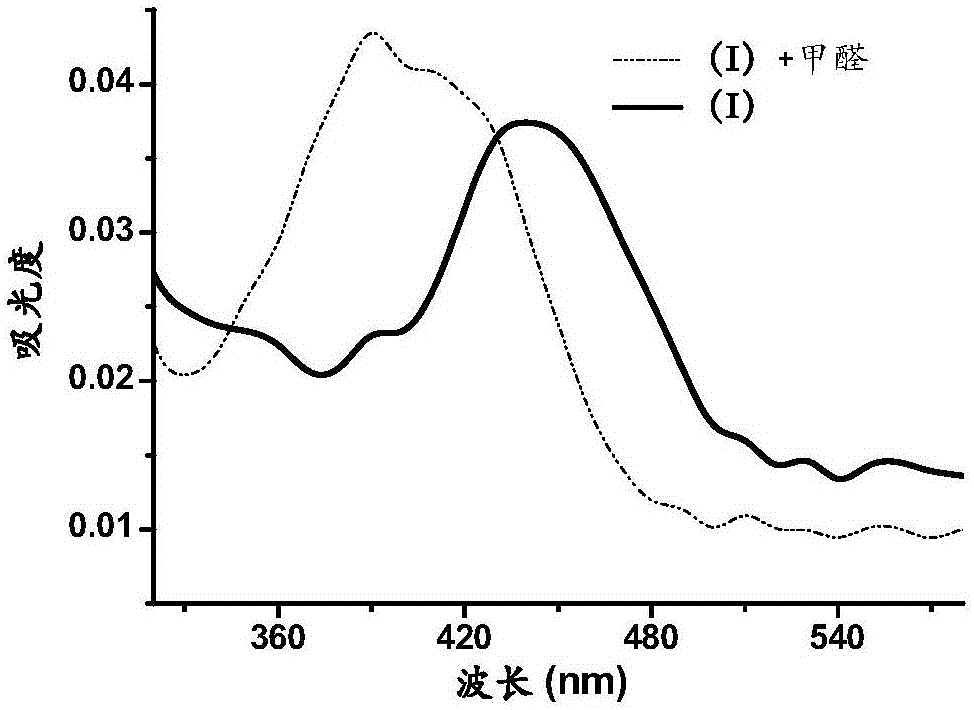
[0037] Embodiment 2 probe (I) adds the ultraviolet absorption spectrometry of 0mM and 5mM formaldehyde concentration under the condition of pH 7.4
[0038]Accurately weigh a certain amount of probe (I), prepare a probe stock solution with a concentration of 1mM with dimethyl sulfoxide, pipette 2μL into 394μL phosphate buffer (10mM pH=7.4), add 4μL respectively Ultrapure water and 4μL, 500mM formaldehyde aqueous solution were reacted at 37°C for 3 hours, and the ultraviolet absorption spectra of the two mixed solutions were measured. The results are shown in image 3 .
[0039] Experiments have shown that the difference in the ability of the 3-position of naphthalimide dyes to push up and pull electrons can affect the absorption spectrum of the compound. Since the aldehyde group has a relatively strong electron-pull ability, the product of the reaction between probe (I) and formaldehyde The absorption spectrum has a blue-shift process, and the maximum absorption wavelength dro...
Embodiment 3
[0040] Example 3 Detection of the fluorescence effect of probe (I) under the condition of pH 7.4 and excitation wavelength 450nm by adding different equivalent formaldehyde concentrations.
[0041] Accurately weigh a certain amount of probe (I), prepare a probe stock solution with a concentration of 1mM with dimethyl sulfoxide, pipette 2μL into 394μL phosphate buffer (10mM pH=7.4), add 4μL respectively Formaldehyde aqueous solutions with different concentrations (final concentration of formaldehyde in water are 0mM, 0.25mM, 0.5mM, 1mM, 2mM, 5mM respectively) were reacted at 37°C for 3 hours, and their fluorescence values were measured. The excitation wavelength is 450nm, the emission wavelength is 480-740nm, see the fluorescence spectrum Figure 4 .
[0042] Experiments have shown that when the formaldehyde equivalent is increased, due to the strong electron-pulling ability of the aldehyde group, when the excitation wavelength is 450nm, the emission spectrum of the product ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com