Positive quality control product for detecting HBB/GJB2/ATP7B/PAH genetic disease genes and preparation method of positive quality control product
A positive quality control substance and positive plasmid technology, applied in the fields of molecular biology and medical testing, can solve the problems of inability to truly simulate human genomic DNA, inability to cover other regions of genomic DNA, inappropriate sequencing, etc. Specific quality control, easy-to-prepare results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Embodiment 1: the establishment of positive quality control product preparation method
[0057] (1) Two cases of wild type Human Genome DNA (WHGD) acquisition:
[0058] WHGD was extracted from wild-type human genome peripheral whole blood sample A and sample B according to the operating instructions of the QIAamp DNA Blood Mini Kit kit (QIAGEN, 69504).
[0059] (2) Acquisition of mutation-positive plasmids:
[0060] 1) Insert design and synthesis:
[0061] Using bioinformatics related technology to design the insert sequence as:
[0062] ATGGACGTGCTCATCGTCCTGGCCACAAGCATTGCTTATGTTTATTCTCTGGTCATCCTGGTGGTTGCTGTGGCTGAGAAGGCGGAGAGGAGCCCTGTGACATTCTTCGACACGCCCCCCATGCTCTTTGTGTTCATTGCCCTGGGCC GTGGCTGGAACACTTGGCAAAGGTAACAGCAGCTTCAGGTTCAGAAAAGAGCTGCTCCTTCAGTAAACAAAATCTCACTTCCTCTGAACACCATGTTTAGAATTACTAATTATAC ATCTGGCTCACCGTCCTTCTTCATTTTTCGCATTATGATCC TCGTTGTGGCTGCAAAGGAGGTGTGGGGAGATGAGCAGGCCGACTTTGTCTGCAACACCCTGCAGCCAGGCTGCAAG AACGTGTGCTACGATCACTACTTCCCCATCTCCCACATCCGGCTAT...
Embodiment 2
[0104] Embodiment 2: the verification of positive quality control product
[0105] (1) Library construction:
[0106] According to the genetic disease-related HBB / GJB2 / ATP7B / PAH gene mutation detection kit, library construction was performed on the 6 cases of positive quality control products prepared in Example 1, and the constructed library was quantitatively used for sequencing.
[0107] (2) On-machine sequencing:
[0108] Template preparation and template enrichment were performed according to the kit instructions of Ion PGM HiQ OT2 Reagents 200 (Life Technologies, A26428) and Ion PGMHiQ OT2 Solutions 200 (Life Technologies, A26429).
[0109] On-machine sequencing was performed according to the kit instructions of Ion PGM HiQ Sequencing 200 Reagents (Life Technologies, A26431), IonPGM HiQ Sequencing 200 Solutions (Life Technologies, A26430) and Ion PGMSequencing Nucleotides (Life Technologies, A26432).
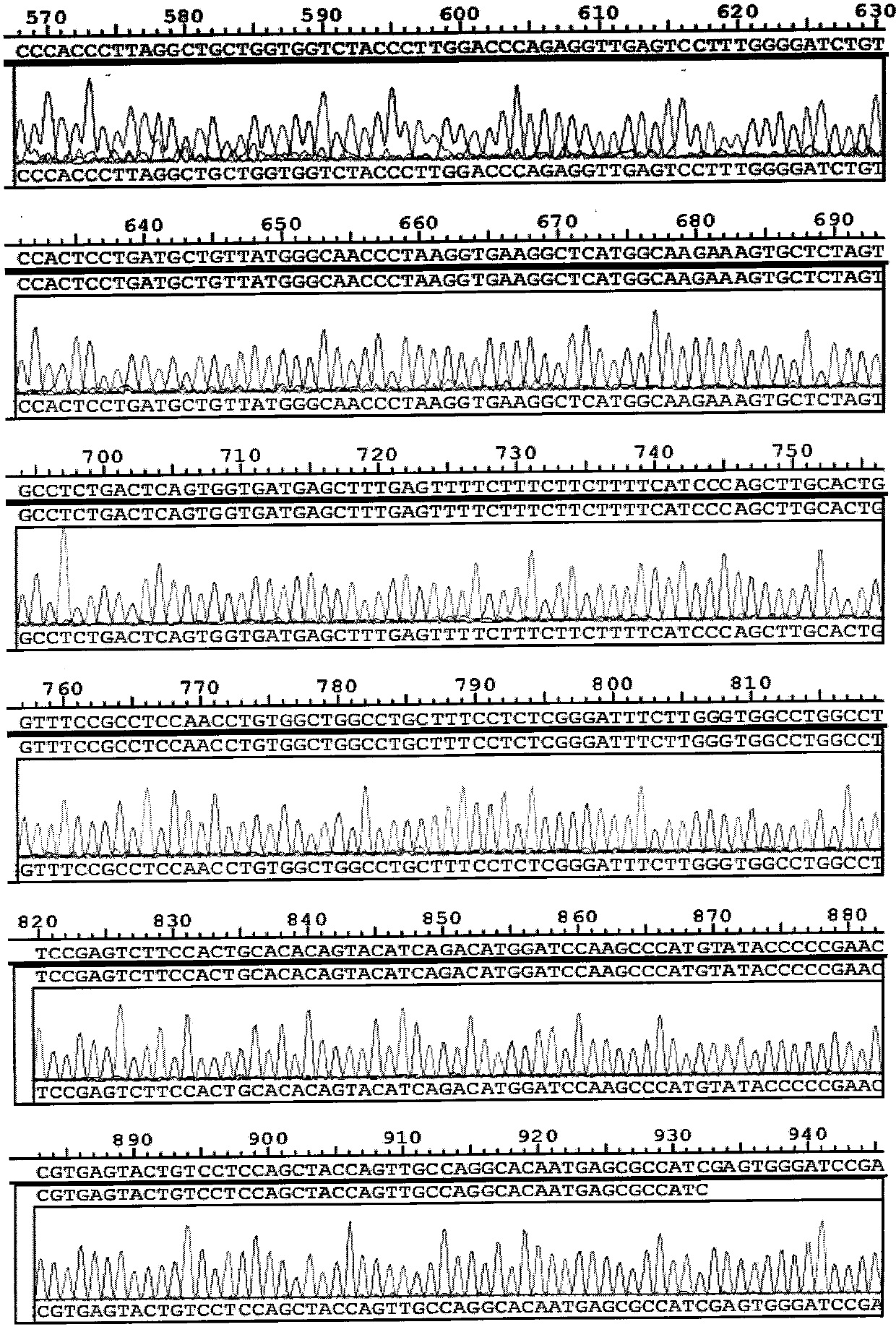
[0110] (3) Results:
[0111] Using biological information technolo...
Embodiment 3
[0115] Embodiment 3: repeatability test verification
[0116] (1) Reproducibility verification of the preparation method of positive quality control products
[0117] 1. Experiment:
[0118] Select 5 cases of wild-type human peripheral whole blood samples to extract genomic DNA, and operate according to the steps of Example 1 and Example 2 with the mutation-positive sequences to obtain 5 cases of positive quality control products, respectively PC-1, PC-2, and PC-3 , PC-4, PC-5, according to the operation steps of Example 2, these 5 cases of positive quality control products were experimentally verified.
[0119] 2. Results:
[0120] Using biological information technology for data analysis, the sequencing results of PC-1 / 2 / 3 / 4 / 5 are shown in the table below:
[0121]
[0122] The sequencing depth values corresponding to the 4 mutation sites of the 5 quality controls in the above table are all ≥ 20, and the mutation frequency is 40% to 65%. The coefficient of variation ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com