Magnetic particle-based quantitative chemiluminescent assay kit for anti-double-stranded DNA antibody IgG, and preparation and detection methods thereof
A technology of magnetic particles and reagent kits, applied in chemiluminescence/bioluminescence, analysis by causing chemical reactions of materials, measurement devices, etc., can solve problems such as applications that have not been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
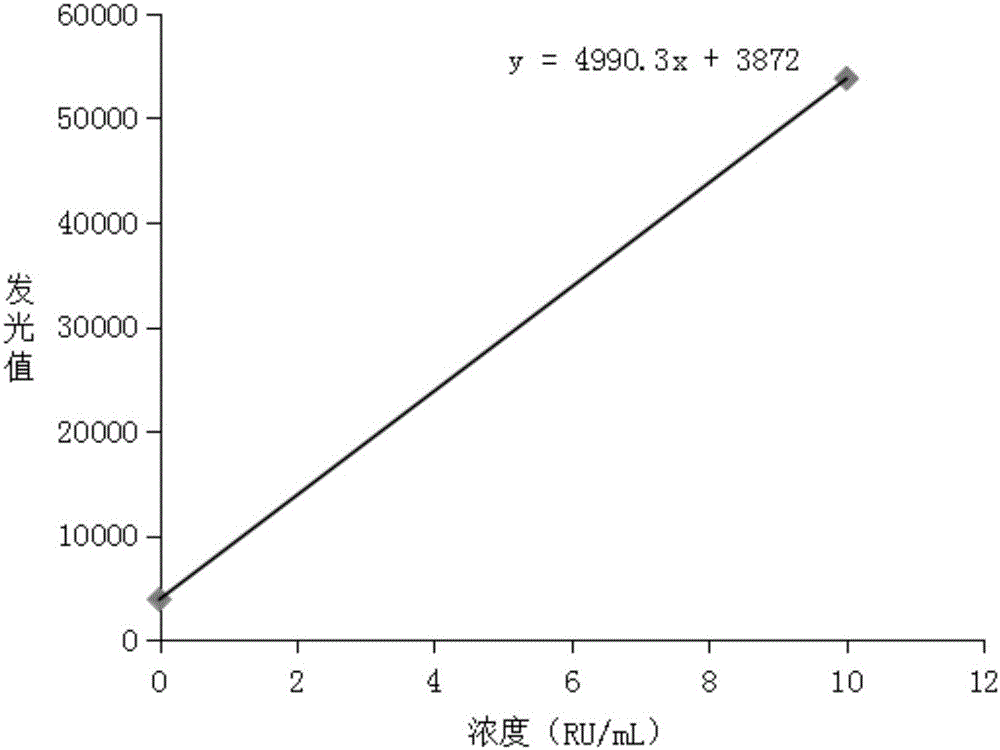
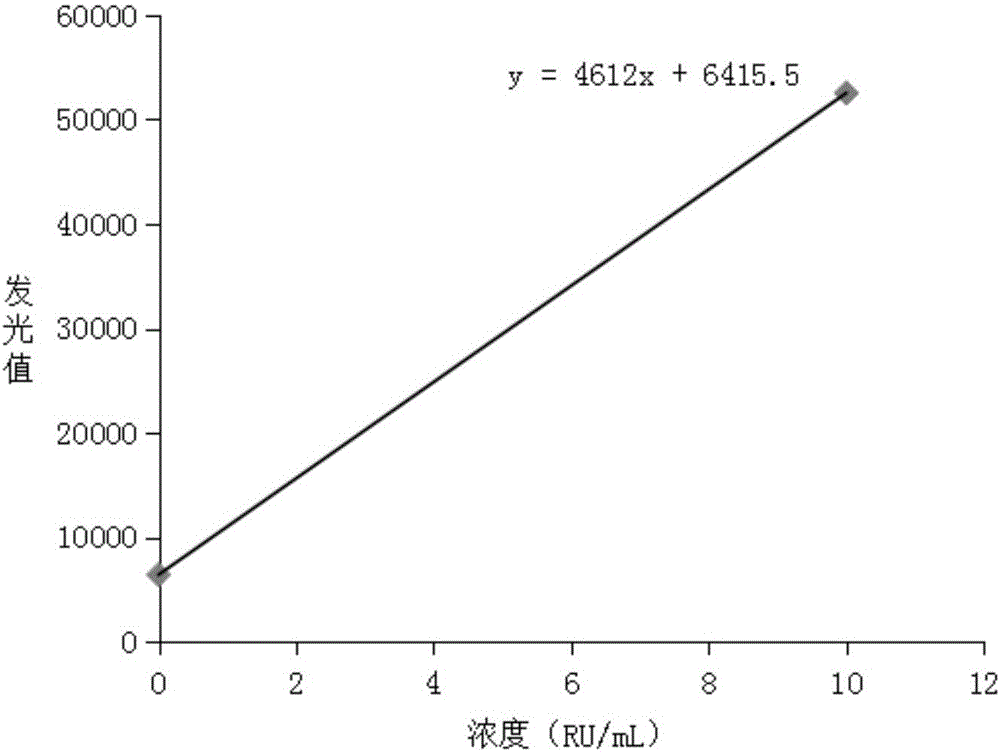
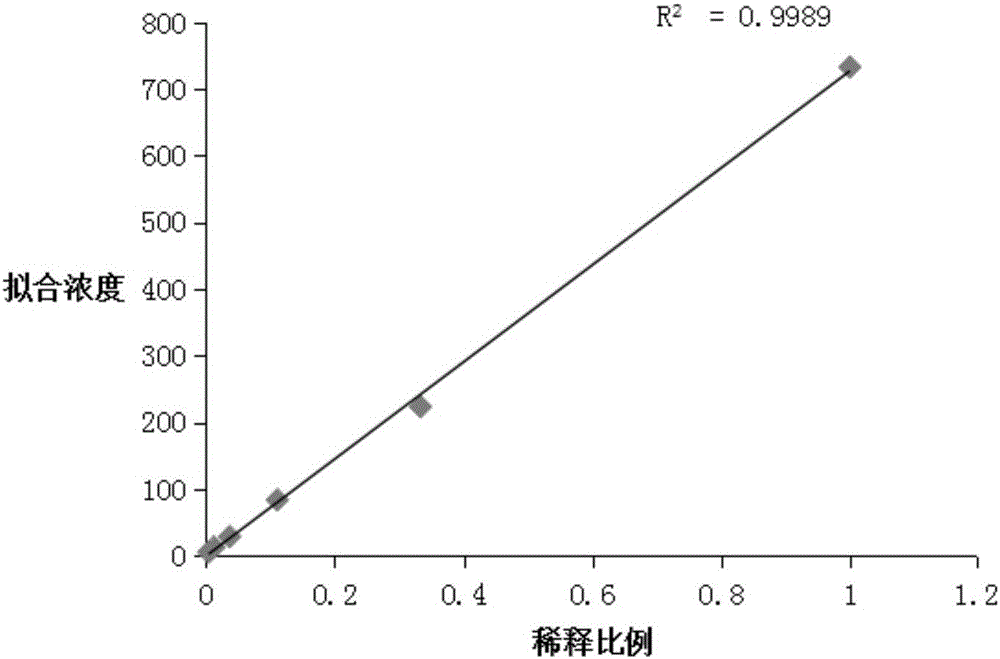
Image
Examples
Embodiment 1
[0066] Preparation of anti-double-stranded DNA antibody IgG calibrator:
[0067] a. Prepare anti-double-stranded DNA antibody IgG calibrator dilution:
[0068] Add 800ml of purified water, 11.2g of Tris, 8.6g of sodium chloride and 2ml of Proclin300 into the container, stir well until completely dissolved; use 4M HCL to adjust the pH of the solution to 7.0-7.5; add 40g of bovine serum albumin Add to the container, stir well until completely dissolved; then adjust the pH of the solution to 7.0-7.5 with 4M HCL; dilute the solution to 1L with purified water, and filter with a 0.2μm filter to obtain the anti-double-stranded DNA antibody IgG calibrator Diluent, store at 2-8°C until use;
[0069] b. Prepare anti-double-stranded DNA antibody IgG calibrator:
[0070] Dilute anti-double-stranded DNA antibody IgG with anti-double-stranded DNA antibody IgG calibrator diluent to each concentration point of 0, 10, 100, 200, 400, 800 IU / mL.
Embodiment 2
[0072] Preparation of anti-double-stranded DNA antibody IgG quality control:
[0073] Dilute the anti-double-stranded DNA antibody IgG with the above-mentioned anti-double-stranded DNA antibody IgG calibrator diluent to 100, 400 IU / mL at each concentration point.
Embodiment 3
[0075] Preparation of Reagent No. 1:
[0076] a. Prepare reagent No. 1 dilution:
[0077] Add 800ml of purified water, 12.1g of Tris, 5.8g of sodium chloride and 2ml of Proclin300 into the container, stir well until completely dissolved; add 5g of bovine serum albumin into the container, stir well until completely dissolved; dissolve the solution with 4M HCL Adjust the pH value of the solution to 7.0-7.5; dilute the solution to 1L with purified water, filter it with a 0.2μm filter to obtain the No. 1 dilution of the reagent, and store it at 2-8°C for later use;
[0078] b. Prepare reagent No. 1:
[0079] Dissolve the double-stranded DNA antigen in purified water, dialyze it with a carbonate buffer solution with a concentration of 0.2M and a pH of 9.0 for 2 hours at 2-8°C, and then concentrate it to an antigen solution with a concentration of 2-4 mg / mL. Prepare a biotin solution with a concentration of 0.5-1.0mg / ml in a carbonate buffer solution of 0.2M and pH 8.5-9; prepare ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com