Sodium-ion battery oxide cathode material, and preparation method and application thereof
A sodium-ion battery and positive electrode material technology, applied in battery electrodes, secondary batteries, electrochemical generators, etc., can solve the problems of no performance, inability to provide high-voltage area capacity, and inability to perform stably
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
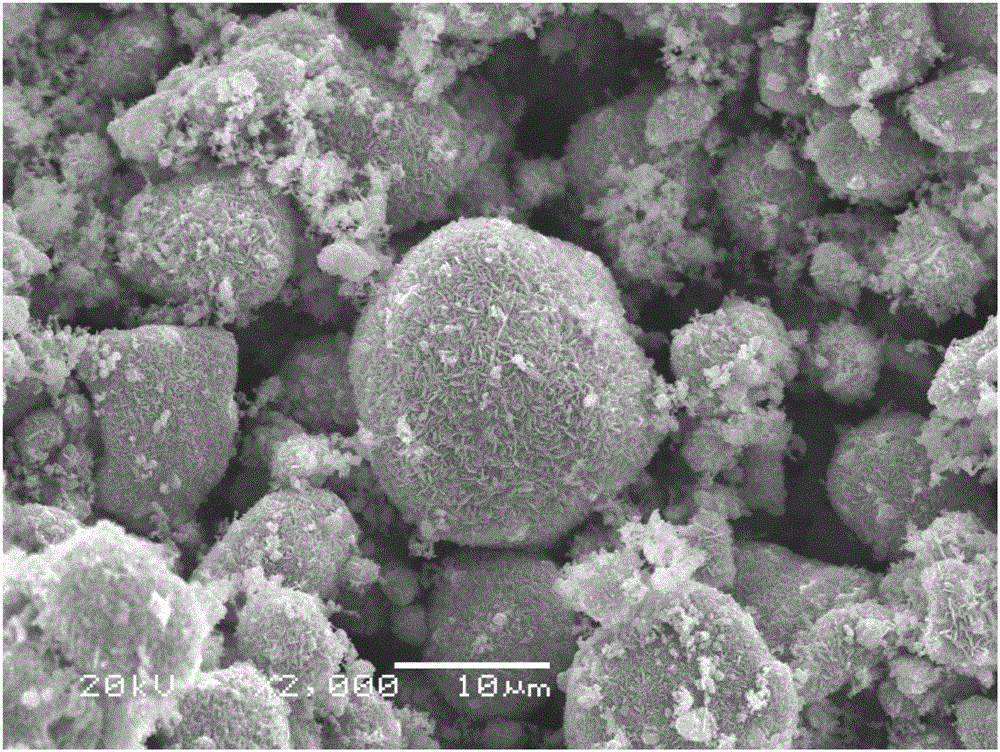
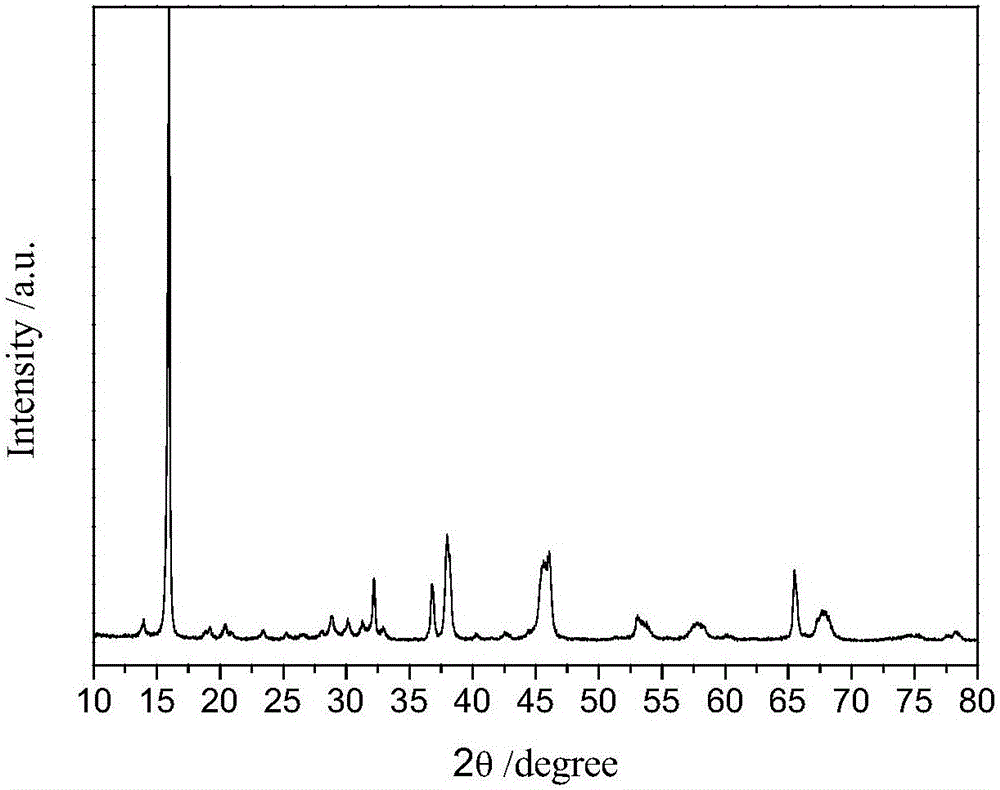
[0029] Will Li 2 CO 3 , Na 2 CO 3 ,MnO 2 Mix evenly at a molar ratio of 1:3:8, then grind evenly in an agate mortar, heat up to 600°C in a muffle furnace at a rate of 2°C / min and keep it for 24 hours, then cool with the furnace to obtain Li 0.25 Na 0.75 MnO 2 . Particle size is 5-20um, spherical particles (such as figure 1 ), XRD shows that the material is an obvious layered structure ( figure 2 ). After mixing the material with acetylene black and polyvinylidene fluoride (PVDF) at a ratio of 8:1:1, add the organic solvent N-methylpyrrolidone (NMP) as a dispersant, and coat the material evenly on the aluminum foil Then put it into an oven at 120°C and bake for 6-12 hours, then punch it into a positive electrode piece with a diameter of 14mm. Use metal sodium sheet as the negative electrode, whatman GF / D as the diaphragm, and 1mol / L NaClO 4 PC: DMC: FEC = 49: 49: 2v% is the electrolyte, and the battery is installed in a glove box filled with high-purity argon gas wi...
Embodiment 2
[0031] Will Li 2 CO 3 , Na 2 CO 3 ,MnO 2 After mixing evenly at a molar ratio of 1:2:3 and ball milling evenly, raise the temperature in a muffle furnace to 800°C at a heating rate of 2°C / min and keep it for 24 hours, then cool with the furnace to obtain Li 1 / 3 Na 2 / 3 MnO 2 . The battery was assembled according to the method of Example 1, and the charging and discharging test was carried out. The results show that the synthesized material has a reversible charge-discharge voltage platform above 4V when it is charged and discharged for the first time like Example 1, and the platform is higher than that of Li 0.25 Na 0.75 MnO 2 Slightly longer, and gradually shortened with the cycle plateau voltage.
Embodiment 3
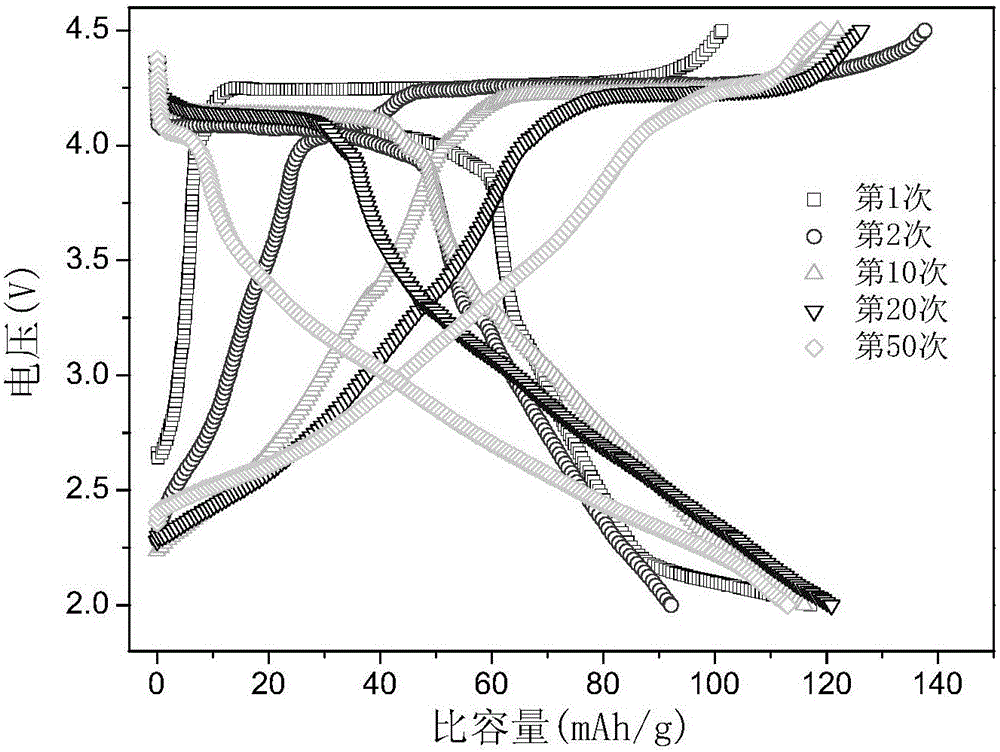
[0033] Will Li 2 CO 3 , Na 2 CO 3 ,MnO 2 , Fe 2 o 3 After mixing evenly at a molar ratio of 1:4:9:0.5 and ball milling evenly, heat up to 750°C in a muffle furnace at a rate of 2°C / min and keep it for 24 hours, then cool with the furnace to obtain Li 0.2 Na 0.8 mn 0.9 Fe 0.1 o 2 . The battery was assembled according to the method of Example 1, and the charging and discharging test was carried out. The result is as Figure 5 : Compared with the voltage curve of Example 1, the synthesized material does not change much when it is first charged, but the plateau changes slightly with cycles.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com