Synthesis method of chidamide
A technology of chidamide and its synthesis method, which is applied in the field of synthesis of chemical drugs, can solve the problems of low purity of chidamide, many impurities, and difficult removal, and achieve the effects of easy control, high product purity, and less impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
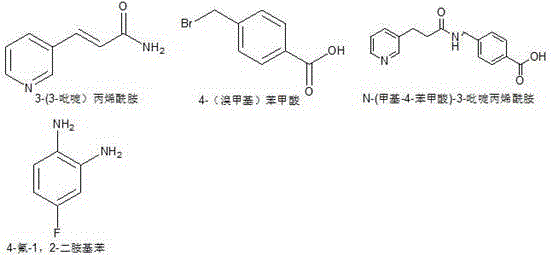
[0032] (1) Dissolve 14.8g of 3-(3-pyridine)acrylamide in 148g of DMF, stir until dissolved, add 23.7g of 4-(bromomethyl)benzoic acid in small batches under ice bath, and heat up to React at 50°C until dissolved, then stir for 1 hour;
[0033] (2) Adjust the pH to 14 with sodium hydroxide, and control the temperature below 15°C. The reaction solution was poured into ice water, filtered immediately to obtain intermediate 1, and dried at a constant temperature of 70 degrees Celsius for 4 hours to obtain intermediate 1 with a dry weight of 25.1 g and a molar yield of 89%;
[0034] (3) Dissolve 25.1 g of intermediate 1 obtained in the previous step in 201 g of tetrahydrofuran, add tetrahydrofuran hydrochloric acid gas dropwise under ice bath, and adjust the pH to 7.
[0035] (4) Add 25.1 g of trifluoroacetic acid dropwise under ice bath and protect it with nitrogen gas. After stirring for 15 minutes, add 12.4 g of raw material 3,4-diaminofluorobenzene into the reaction system, sti...
Embodiment 2
[0038] (1) Dissolve 14.8g of 3-(3-pyridine)acrylamide in 148g of DMF, stir until dissolved, add 25.8g of 4-(bromomethyl)benzoic acid in small batches under ice bath, and heat up to React at 50°C until dissolved, then stir for 1 hour;
[0039] (2) Adjust the pH to 14 with sodium hydroxide, and control the temperature below 15°C. The reaction solution was poured into ice water, filtered immediately to obtain intermediate 1, and dried at a constant temperature of 70 degrees Celsius for 4 hours to obtain intermediate 1 with a dry weight of 25.2 g and a molar yield of 89.4%;
[0040] (3) Dissolve 25.2g of intermediate 1 obtained in the previous step in 202g of tetrahydrofuran, add tetrahydrofuran hydrochloric acid gas dropwise under ice bath, and adjust the pH to 7;
[0041] (4) 25.2 g of trifluoroacetic acid was added dropwise under ice bath and protected by nitrogen gas. After stirring for 15 minutes, 12.4 g of raw material 3,4-diaminofluorobenzene was added to the reaction syst...
Embodiment 3
[0044] (1) Dissolve 14.8g of 3-(3-pyridine)acrylamide in 148g of DMF, stir until dissolved, add 23.7g of 4-(bromomethyl)benzoic acid in small batches under ice bath, and heat up to React at 50°C until dissolved, then stir for 1 hour;
[0045] (2) Adjust the pH to 14 with sodium hydroxide, and control the temperature below 15°C. The reaction solution was poured into ice water, filtered immediately to obtain intermediate 1, and dried at a constant temperature at 70°C for 4 hours to obtain intermediate 1 with a dry weight of 25.1 g and a molar yield of 89%.
[0046] (3) Dissolve 25.1 g of intermediate 1 obtained in the previous step in 201 g of tetrahydrofuran, add tetrahydrofuran hydrochloric acid gas dropwise under ice bath, and adjust the pH to 7;
[0047] (4) Add 25.1 g of trifluoroacetic acid dropwise under ice bath and protect with nitrogen gas. After stirring for 15 minutes, add 12.4 g of raw material 3,4-diaminofluorobenzene into the reaction system, and stir at 25°C for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com