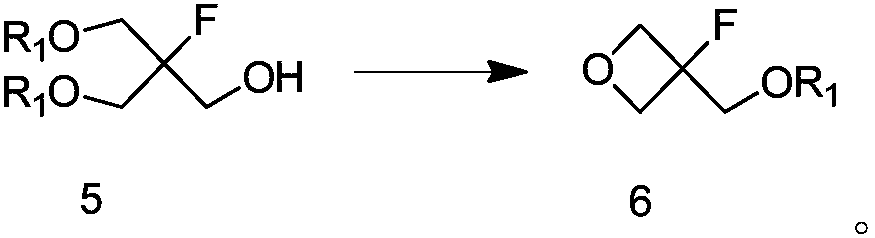
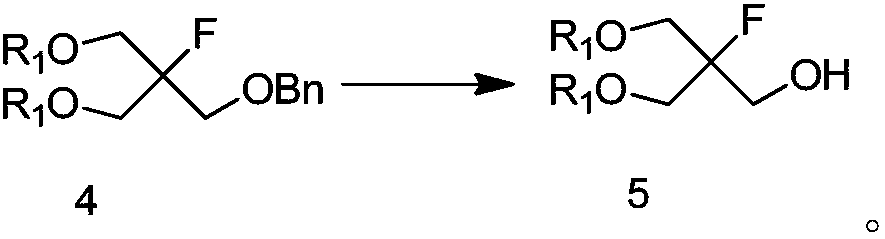
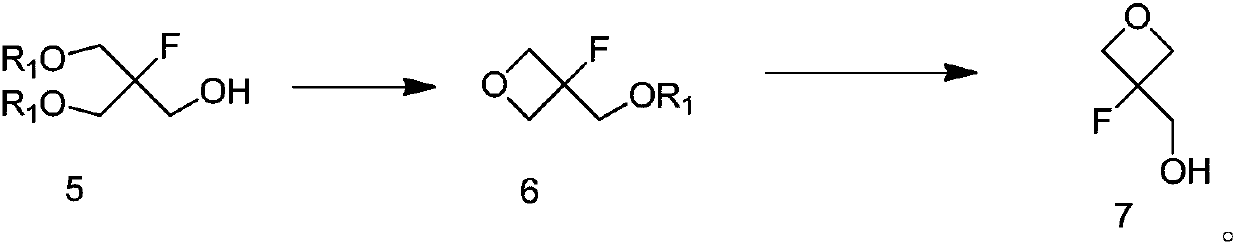
Method for preparing 3-fluorooxetane-3-methanol and intermediate of 3-fluorooxetane-3-methanol
A technology for fluorooxetane and intermediates, which is applied in the field of preparation of 3-fluorooxetane-3-methanol and its intermediates, and can solve the problems of unsuitable scale-up production, high total cost, and environmental pollution. friendly questions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Sodium hydride (3.6 g, 0.15 mol) was added to tetrahydrofuran (120 mL) in batches, the temperature was lowered to -10 °C to -5 °C, the temperature was controlled at -10 °C to -5 °C, and diethyl fluoromalonate ( 15g, 0.084mol), the dropwise addition was completed in 3 to 4 hours. The system turned gray at night, and was stirred at -10°C to -5°C for half an hour. Chloromethyl benzyl ether (16.4 g, 0.1 mol) was added dropwise, the temperature was controlled at -10°C to -5°C, and the dropwise addition was completed in 4 to 6 hours. After dripping, the mixture was kept at -10°C to -5°C and stirred for 1 hour, then sampled and sent to TLC, and the reaction was completed. Saturated brine was added to the system, stirred for half an hour, the layers were separated, and the aqueous phase was extracted once with ethyl acetate. The organic phases were combined and washed with saturated sodium bicarbonate, dried over anhydrous sodium sulfate, filtered with suction, and the filtra...
Embodiment 2
[0085] 2-Fluoro-2-benzyloxymethyl-malonate diethyl ester (20 g, 0.067 mol) was dissolved in tetrahydrofuran (220 mL), and after stirring uniformly, sodium borohydride (4.2 g, 0.11 mol) was added, and the temperature was controlled. 30 ℃~45 ℃, after adding, stirring for 1 hour, sampling and sending to TLC, after the reaction is completed, add water to the system, after stirring for half an hour, concentrate until there is an off-white solid to separate out, add ethyl acetate, adjust the pH with 1 mol / L hydrochloric acid =1-2, separated, the aqueous phase was extracted twice with ethyl acetate, the organic phases were combined, the pH value was adjusted to 7-8 with 50% sodium hydroxide solution, the organic phase was washed with water, washed with saturated brine, and anhydrous sulfuric acid Dry over sodium and concentrate to dryness to give 2-fluoro-2-benzyloxymethyl-propanediol (13 g, 0.061 mol) in 90% yield.
Embodiment 3
[0087] Dissolve 2-fluoro-2-benzyloxymethyl-propanediol (10 g, 0.0467 mol) in tetrahydrofuran (120 mL), add diisopropylethylamine (14.1 g, 0.11 mol), stir evenly, and control the temperature to 0~ Add p-toluenesulfonyl chloride (21.2 g, 0.11 mol) dropwise at 5°C, after the addition, stir for half an hour and sample for TLC, the reaction is complete; add saturated brine to quench the reaction, separate layers, and extract the aqueous phase with ethyl acetate for 2 Second, the organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate, concentrated to dryness, crystallized with petroleum ether, filtered, and dried to obtain 2-(benzyloxymethyl)-2-fluoro-bis(p-methyl) benzenesulfonic acid)-1,3-propanediol ester (20.7 g, 0.0397 mol) in 85% yield.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com