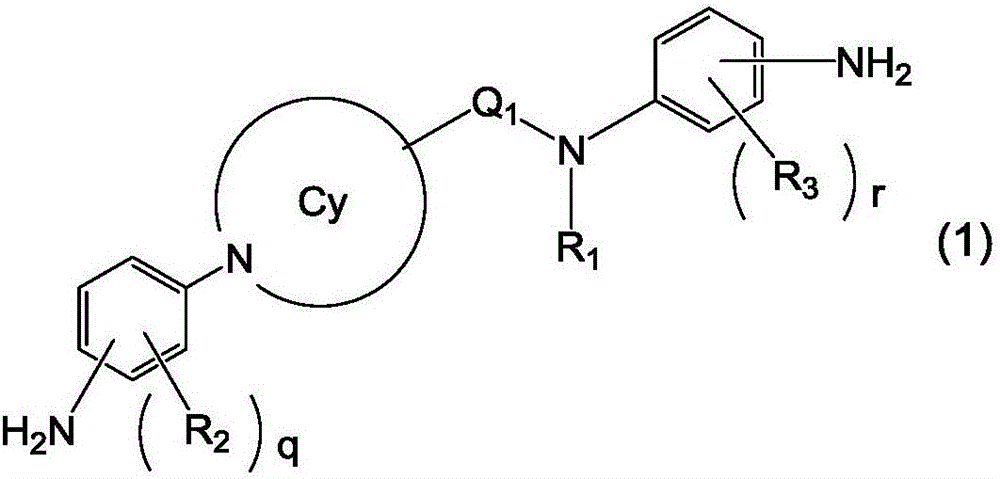
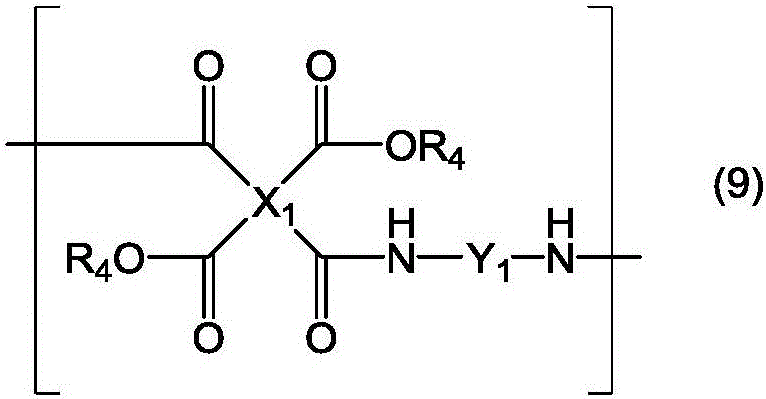
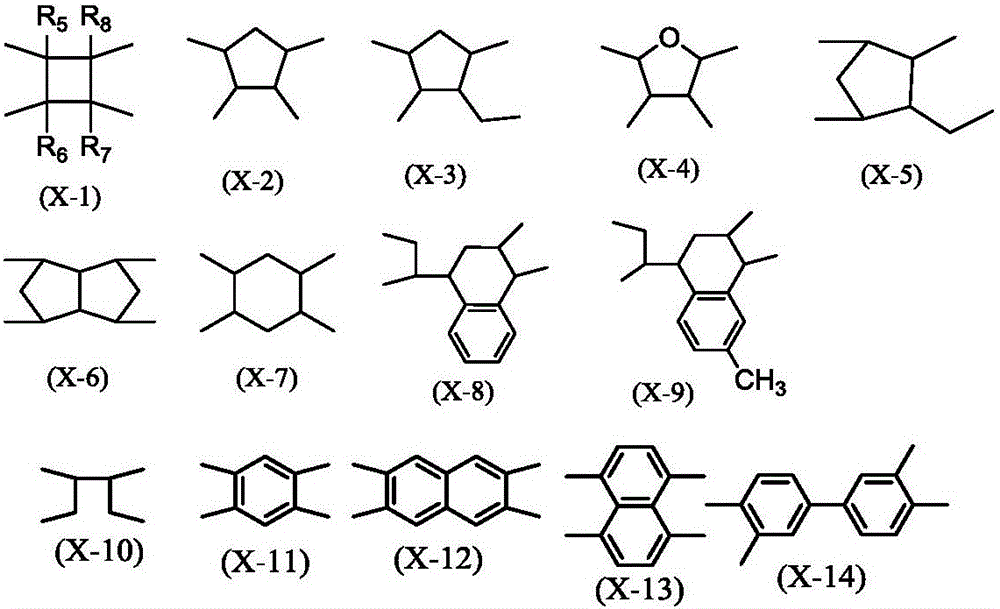
Novel liquid crystal orientation agent, diamine, and polyimide precursor
A polyimide precursor, liquid crystal aligning agent technology, applied in liquid crystal materials, chemical instruments and methods, instruments, etc., can solve the problems of insufficient liquid crystal recovery, reduced contrast, close distance between pixel electrodes and general electrodes, etc. The effect of high voltage holding ratio and brush abrasion resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0268] Synthesis of (DA-1)
[0269]
[0270]Under a nitrogen atmosphere, dimethylformamide (390 g), 4-fluoronitrobenzene (65.0 g, 0.461 mol), 4-aminomethylpiperidine (25.0 g, 0.219 mol) and carbonic acid were added to a four-necked flask. Potassium (90.9 g, 0.658 mol) was reacted at 60°C. After heating and stirring for 22 hours, the disappearance of the intermediate was confirmed by HPLC. Thereafter, potassium carbonate was removed by filtration, and potassium carbonate was washed twice with 250 g of dimethylformamide. The obtained solution was distilled off under reduced pressure until the content became 295 g, and then 1.50 kg of water was added to precipitate the compound (11). Thereafter, the precipitate was collected by filtration and dried to obtain a crude product of compound (11). The obtained crude product was purified by recrystallization from tetrahydrofuran to obtain compound (11) (58.8 g, 0.165 mol, yield: 75.3%) as a yellow solid.
[0271] HPLC measurement...
Embodiment 2
[0286] Synthesis of (DA-2)
[0287]
[0288] Under a nitrogen atmosphere, tetrahydrofuran (534 g), compound (11) (21.4 g, 0.0601 mol) obtained by (Example 1) and potassium tert-butoxide (8.10 g, 0.0722 mol) were added to a four-necked flask. Methyl iodide (9.35 g, 0.0660 mol) was added dropwise to the stirred solution, and the temperature was raised to 40°C. After reacting for 24 hours, methyl iodide (10.2 g, 0.0717 mol) and potassium tert-butoxide (2.98 g, 0.0266 mol) were further added, and the disappearance of the raw materials was confirmed by HPLC. Thereafter, 100 g of water was added to terminate the reaction. To the crude product obtained by distilling off the reaction liquid under reduced pressure, 500 g of water was added, stirred for 24 hours in a slurry state, and the solid was filtered and dried. Thereafter, recrystallization was performed from tetrahydrofuran to obtain compound (13) (17.4 g, 0.0470 mol) as a yellow solid in a yield of 78.2%.
[0289] 1 H-NM...
Embodiment 3
[0307] Measure 1.98 g (5.00 mmol) of DA-1 into a 50 mL four-neck flask equipped with a stirring device and a nitrogen gas introduction tube, add 20.8 g of NMP, and stir while feeding nitrogen to dissolve it. While stirring this diamine solution, 1.03 g (4.75 mmol) of acid dianhydride (A) was added, and 5.20 g of NMP was further added, and stirred at 23° C. for 3 hours under a nitrogen atmosphere to obtain a polyamic acid solution (PAA-1) . The viscosity of this polyamic acid solution at a temperature of 25° C. was 134 mPa·s.
[0308] Take 7.92 g of the polyamic acid solution (PAA-1) obtained in Synthesis Example 2 into a 100 mL Erlenmeyer flask with a stirring bar, add 2.56 g of NMP, 3-glycidoxypropyltriethoxysilane The liquid crystal aligning agent (A-1) was obtained by stirring 0.67g of 1 mass % NMP solution, BCS3.72g with the magnetic stirrer for 2 hours.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com