A kind of larotaxel water-soluble powder injection and application thereof
A technology of water-soluble powder and larotaxel, which is applied in powder delivery, organic active ingredients, medical preparations of non-active ingredients, etc., and can solve the research on preparations related to nano-polymer micelles of larotaxel that have not been reported. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
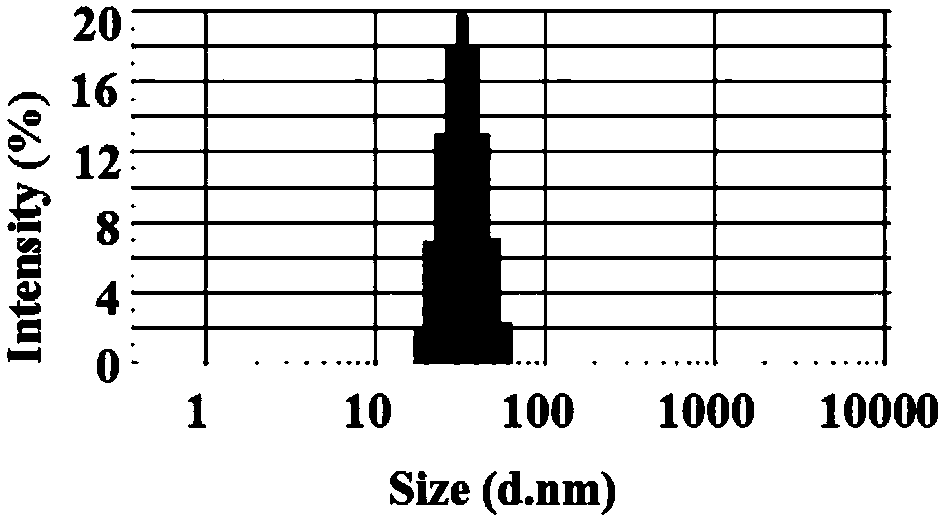
[0031] Weigh 180mg carrier material (mPEG 2000 -PDLLA 1700 ) and 20 mg of larotaxel were placed in a round bottom flask, 10 mL of absolute ethanol was added, and the carrier material and the drug were fully dissolved by ultrasonication. The solution was placed in a rotary evaporator at 40° C. for 60 minutes to evaporate the organic solvent completely to obtain a dry and transparent mixed film matrix. Then add 10mL of water for injection preheated at 60°C, hydrate to form micelles under stirring conditions, add mannitol, first pass through a 0.45μm filter membrane for coarse filtration, and then pass through a 0.22μm sterile filter membrane for fine filtration to sterilize, the filtrate freeze-dried to obtain a sample.
Embodiment example 2
[0033] Weigh 150mg carrier material (mPEG 2000 -PCL 2000 ) and 50 mg of larotaxel were placed in a lyophilized bottle, 5 ml of tert-butanol was added, and the carrier material and the drug were fully dissolved by ultrasonication. The solution is lyophilized and the organic solvent is completely removed to obtain a mixed matrix of drug and carrier material. Then add 10mL of water for injection preheated at 60°C, hydrate to form micelles under stirring conditions, add sucrose, first pass through a 0.45μm filter membrane for coarse filtration, and then pass through a 0.22μm sterile filter membrane for fine filtration to sterilize, and the filtrate is frozen Dry to get a sample.
Embodiment example 3
[0035] Weigh 180mg carrier material (mPEG 5000 -PGA 6000 ) and 20 mg of larrotaxel were placed in a round bottom flask, 5 ml of tetrahydrofuran was added, and the carrier material and the drug were fully dissolved by ultrasonication. The solvent was distilled under reduced pressure to completely remove the organic solvent to obtain a dry and transparent mixed film matrix. Then add 10mL of water for injection preheated at 50°C, vortex for 3 minutes, add trehalose, pass the hydration solution through a 0.45 μm filter membrane for coarse filtration, and then pass through a 0.22 μm filter membrane for fine filtration to sterilize, and the filtrate is freeze-dried to obtain sample.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com