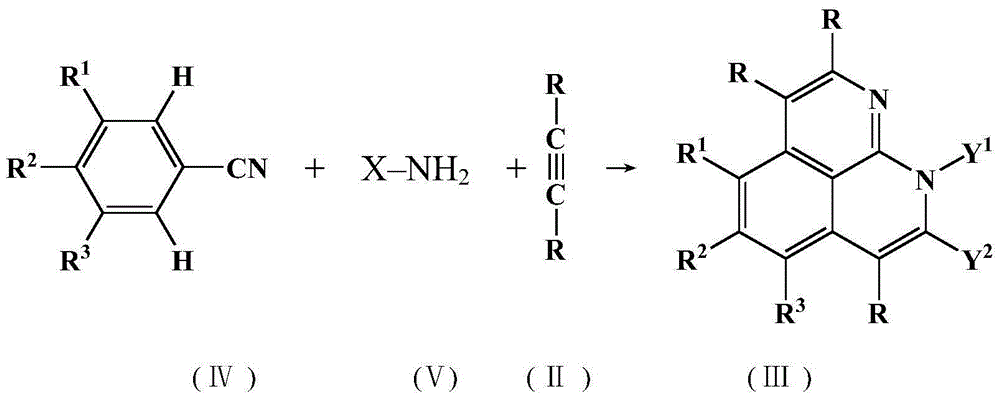Method for manufacturing 1,9-diazaphenalene derivative
A technology of derivatives and diazepam, applied in the field of preparation of 1,9-diazepine derivatives, can solve problems such as unseen synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~23
[0035] 0.25mmol benzamidine (benzamidine) derivative (I), 0.80mmol alkyne compound (II), 6.0mol% [Cp*Rh(CH 3 EN) 3 ](SbF 6 ) 2 (Cp* represents pentamethylcyclopentadienyl, as a catalyst) and 1.12 mmol (4.5 equivalents) of copper (II) acetate (as an oxidant) are placed in a sealed tube, vacuum pumped and nitrogen purge (purge) 3 times , and then injected 3.5mL 2-methyl-2-butanol (2-methyl-2-butanol, t-amylOH, tert-amyl alcohol) (as a solvent) with a syringe under a nitrogen atmosphere, and reacted at 130°C with stirring 18 hours. After the reaction, it was cooled and diluted with 10 mL of dichloromethane, then filtered with a Celite pad and washed with 50 mL of dichloromethane, and finally the filtrate was collected and concentrated in vacuo, and then filtered with a silica gel column The product (Ⅲ) was purified by chromatography [the eluent was hexane-ethyl acetate (5-15%)]. Its reaction formula is as follows:
[0036]
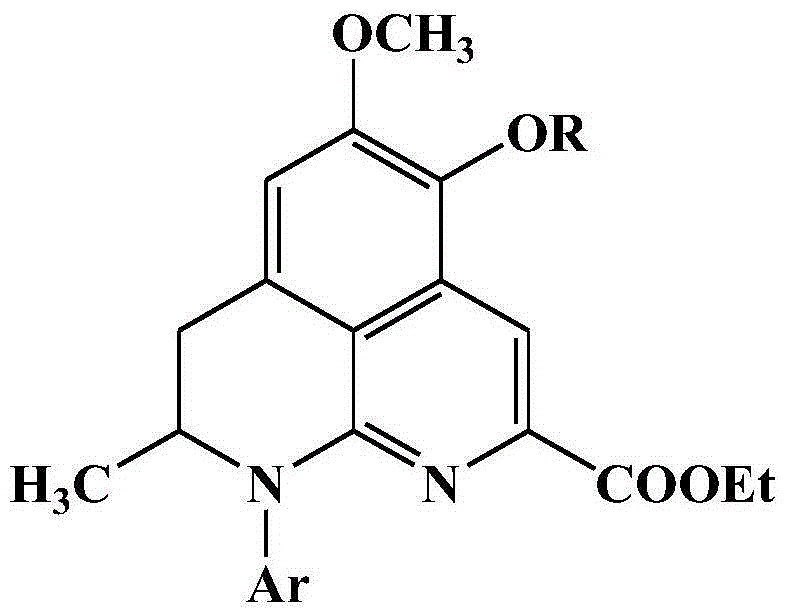
[0037] In the following Examples 1-23, R in th...
Embodiment 1
[0042] 83% yield, scarlet solid, m.p.265-268°C, 1 H NMR (500MHz, CDCl 3 ):δ7.52-7.50(m,5H),7.47-7.38(m,2H),7.28-7.19(m,10H),7.12-7.06(m,5H),7.05-6.96(m,5H),6.92 -6.88(m,2H),6.80(td,J=8.0,2.0Hz,1H),6.50-6.49(m,2H), 13 C NMR (125MHz, CDCl 3 ): δ150.0, 149.3, 140.5, 138.6, 137.8, 137.6, 136.9, 136.8, 135.6, 135.2, 134.6, 131.8, 131.7, 131.2, 130.9, 130.2, 130.0, 129.7, 128.5, 128.9.2, 126 126.5, 125.9, 125.5, 124.9, 123.2, 121.8, 118.9, 117.5, HRMS (FAB + )m / z:MH + C 49 h 32 N 2 Calculated value: 648.2565, found value 648.2563, IR(KBr): 3054, 2929, 2337, 1342cm -1 .
Embodiment 2
[0044] Yield 78%, orange solid, m.p.294-297°C, 1 H NMR (400MHz, CDCl 3 ):δ7.51-7.45(m,5H),7.26-7.17(m,8H),7.07-6.95(m,11H),6.93-6.86(m,3H),6.77(td,J=7.2,1.2Hz ,1H),6.71(s,1H),6.45(d,J=7.2Hz,2H),2.27(s,3H), 13 C NMR (125MHz, CDCl 3 ): δ150.2,149.3,141.4,140.7,138.8,138.0,137.9,137.4,137.1,136.9,135.8,135.2,134.6,131.9,131.8,131.3,130.2,130.1,129.8,129.3,181.127 127.5, 127.0, 126.7, 126.6, 126.5, 125.8, 125.4, 124.9, 122.8, 120.4, 119.0, 118.8, 22.6, HRMS (EI + )C 50 h 34 N 2 Calculated: 662.2722, Found: 662.2724, IR(KBr): 3054, 2931, 2337, 1342cm -1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com