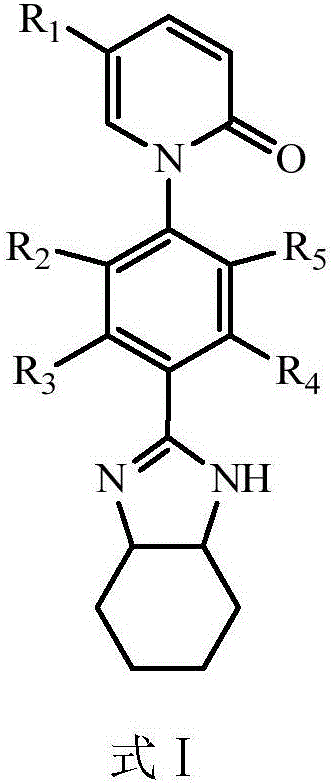
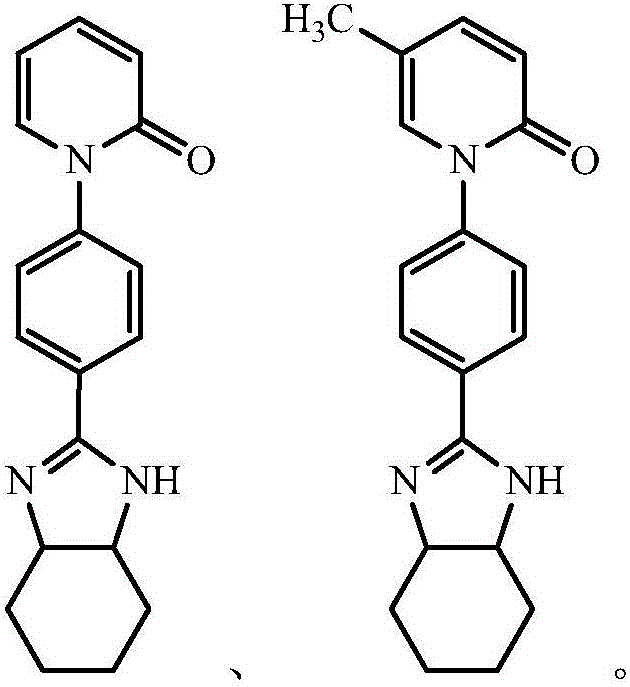
Application of pirfenidone derivative to pharmacy
A drug and pharmacy technology, applied in anti-tumor drugs, drug combinations, pharmaceutical formulations, etc., can solve the problems of no anti-fibrotic drugs, anti-tumor drugs, poor anti-fibrotic activity, etc., and achieve the effect of good industrialization prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
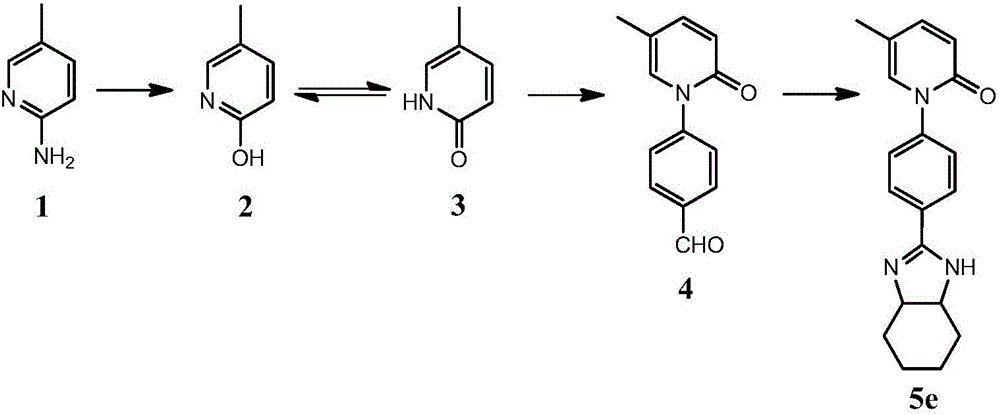
[0040] Embodiment 1, the synthesis of compound 5e of the present invention
[0041] synthetic route:
[0042]
[0043] 1. Synthesis of compound 3 (5-methyl-2-(1H)-pyridone)
[0044] First add 3.40mL of 50% sulfuric acid (v / v) to a 25mL reaction flask, then add 1.00g (10mmol) of 2-amino-5-picoline (compound 1), cool to below 10°C in an ice-salt bath, and stir for several After 10 minutes, the reaction solution turned milky white; then slowly added dropwise 1.72g (25mmol) NaNO 2 with 3mL H 2 The mixed solution composed of O has brown-yellow gas with pungent odor during the dropwise addition process. After the addition, the reaction solution turns light yellow. Use 10% dilute sulfuric acid to adjust the pH to 7-8, reflux and stir for about 20 minutes, and spin Most of the water was removed, an appropriate amount of 300 mesh silica gel was added thereto, spin-dried, poured into a glass sand core funnel, rinsed with ethyl acetate and suction-filtered, and the filtrate was spi...
Embodiment 2
[0053] Embodiment 2, the synthesis of compound 7a of the present invention
[0054] synthetic route:
[0055]
[0056] Using compound 1' (2-aminopyridine) as a raw material, compound 7a was prepared according to a method similar to Example 1, and the single-step yield of the third step was 64%.
[0057] Compound 7a: 1-(4-(3a,4,5,6,7,7a-hexahydro-1H-benzo[d]imidazol-2-yl)phenyl)pyridin-2(1H)-one, yellow Solid, m.p.254-256°C;
[0058] 1 H NMR (400MHz, DMSO) δ8.07 (d, J = 8.6Hz, 2H), 7.83–7.69 (m, 3H), 7.59–7.52 (m, 1H), 6.51 (d, J = 9.3Hz, 1H) ,6.38(td,J=6.7,1.1Hz,1H),3.50–3.37(m,1H),3.08(dd,J=14.5,7.2Hz,1H),2.57(dd,J=11.1,6.1Hz,4H ),1.75(s,1H),1.44(dd,J=16.8,11.7Hz,4H);
[0059] 13 C NMR (101MHz, DMSO) δ163.61, 160.91, 145.35, 141.14, 138.39, 129.26, 127.76, 122.32, 120.64, 106.21, 56.01, 53.84, 31.94, 25.33, 23.97, 18.45;
[0060] HRMS (ESI) calcd for C 18 h 19 N 3 O[M+H] + 294.1607, found 294.1604.
Embodiment 3
[0066] Embodiment 3, the anti-fibrosis activity of the compound of the present invention
[0067] 1. Cell culture
[0068] Inoculate 3T3L1 cells in cell culture medium containing 10% FBS, add 100 IU / mL penicillin and streptomycin, place them in an incubator containing 5% carbon dioxide at 37°C and culture them. After the cells grow confluent, add 0.25% pancreatic Enzyme digestion and passage, the cells of passage 3-10 were used for experiments. Dissolve pirfenidone and the compound of the present invention in DMSO, filter and sterilize through a 0.22 μm filter membrane, store at -20°C, and thaw before use.
[0069] 2. Evaluation of cell proliferation rate / inhibition rate
[0070] 3T3L1 cells were inoculated in 96-well plates with DMEM medium containing 10% FBS, and the concentration was adjusted to 8×10 4 / hole, cultivated in an environment containing 5% carbon dioxide at 37°C for 24h, added three concentrations of the compounds of the present invention, 100, 200, and 400 μ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com