A kind of preparation method of biurea
A technology of biurea and solution, applied in the direction of organic chemistry, etc., can solve the problems of low purity of biurea, long reaction process, high cost, etc., and achieve the effect of reducing the treatment process and treatment cost, long treatment cycle and improving quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
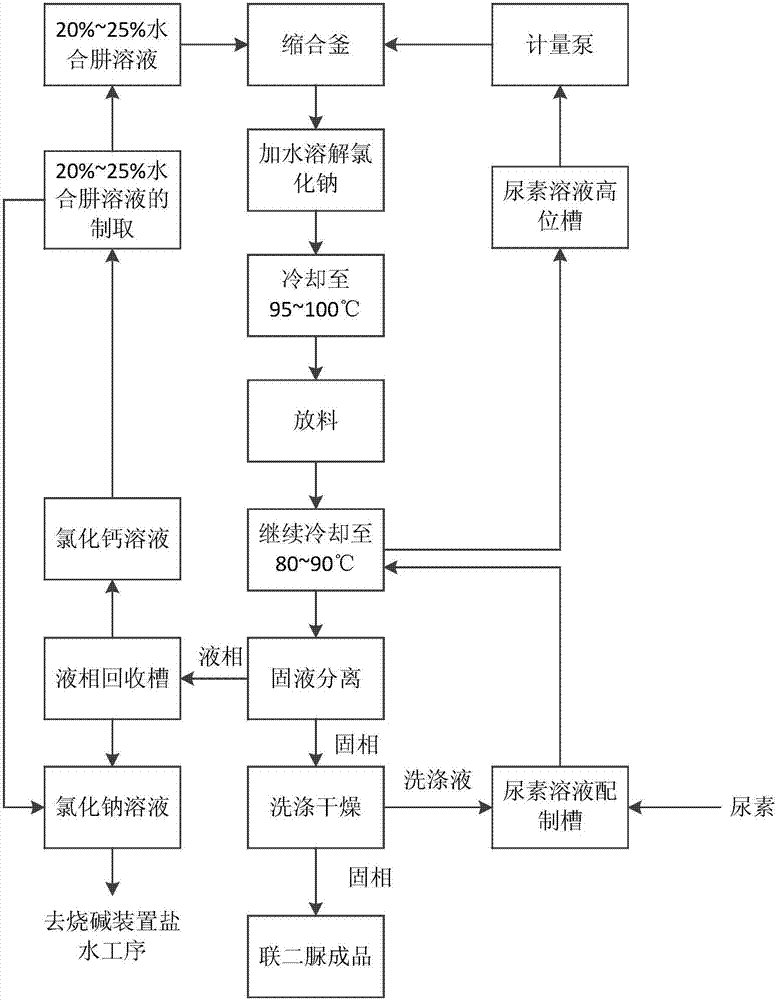
[0047] This embodiment provides a preparation method of biurea, comprising the following steps:
[0048] Step 1: Prepare 20% to 25% hydrazine hydrate solution
[0049] a. The crude hydrazine hydrate solution obtained by the traditional urea method is sampled and analyzed to determine the contents of sodium hydroxide and sodium carbonate in the crude hydrazine hydrate solution respectively, and according to the reaction equation of sodium hydroxide and calcium chloride and sodium carbonate and chlorine The reaction equation of calcium chloride calculates the total mass m of calcium chloride needed to react with sodium hydroxide and sodium carbonate;
[0050] b. input the crude hydrazine hydrate solution into the first stage mixing kettle, and continue to add calcium chloride solid in the first stage mixing kettle, the quality of the added calcium chloride solid is 95% of the total mass m of calcium chloride , the first-stage mixing tank is a mixing tank with a stirrer, the cru...
Embodiment 2
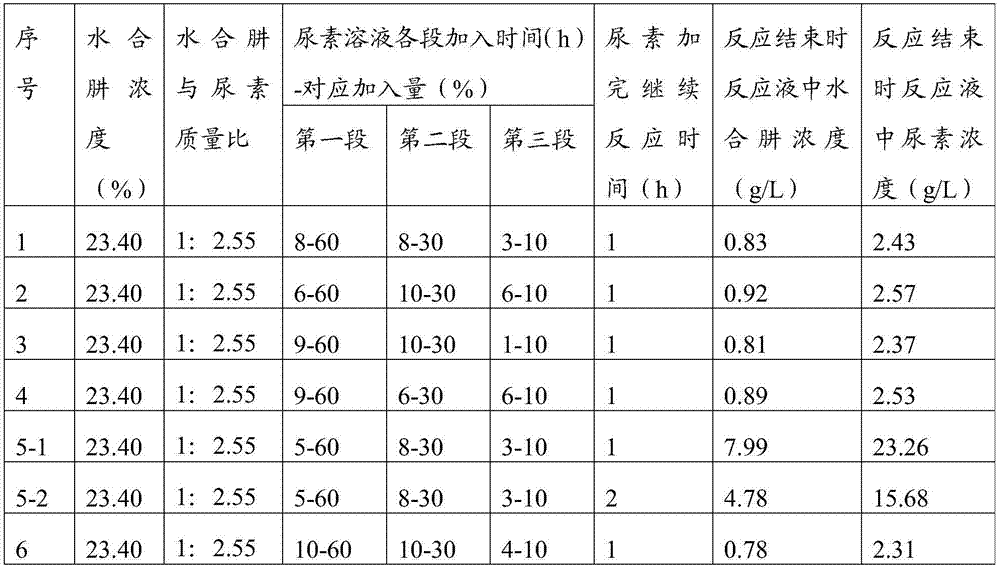
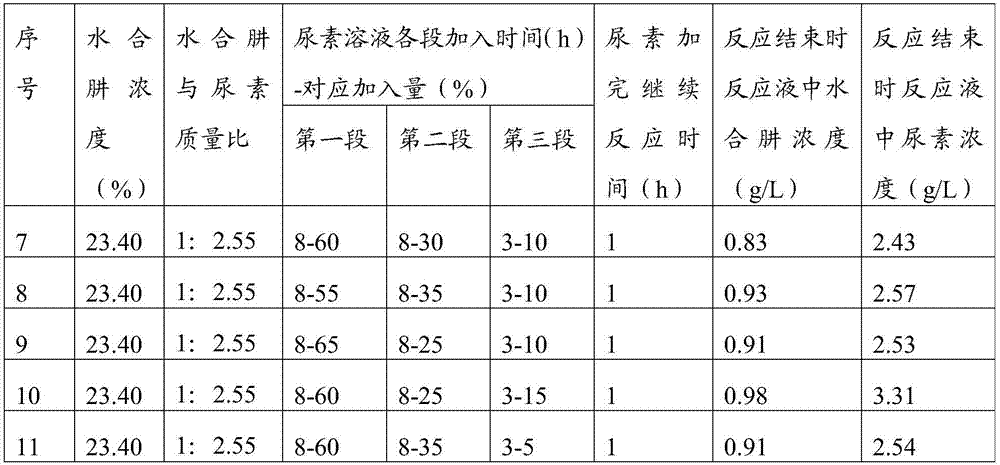
[0064] Prepare biurea according to the steps in Example 1, but add the urea solution according to the following process in step 2: the first section is to add 60% of the total amount of urea solution to the condensation kettle, and the control time is 6h Complete; the second stage is to add 30% of the total amount of urea solution, and the control is completed within 10 hours; the third stage is to add 10% of the total amount of urea solution, and the control time is 6 hours to complete, and the interval between the first stage and the second stage is 2 minutes , the interval between the second section and the third section is 2min.
[0065]After continuing to react for 1 hour through step 3, the concentration of residual hydrazine hydrate in the reaction solution in the condensation tank was sampled and analyzed. After testing, it was 0.92 g / L, and the concentration of residual urea in the reaction solution was 2.57 g / L.
Embodiment 3
[0067] Prepare biurea according to the steps in Example 1, but add the urea solution according to the following process in step 2: the first section is to add 60% of the total amount of urea solution to the condensation kettle, and the control time is 9h Complete; the second stage is to add 30% of the total amount of urea solution, and the control time is 10h to complete; the third stage is to add 10% of the total urea solution, and the control time is 1h to complete, the interval between the first stage and the second stage 0.5min, the interval between the second section and the third section is 1min.
[0068] After continuing to react for 1 hour through step 3, the concentration of residual hydrazine hydrate in the reaction solution in the condensation tank was sampled and analyzed. After testing, it was 0.81g / L, and the concentration of residual urea in the reaction solution was 2.37g / L.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com