Preparation method of 2-halogen-6-alkylthio toluene
A technology of alkylthiotoluene and dimethyl sulfoxide, applied in the field of compound preparation, can solve the problems of high cost, low yield, complicated operation of 2-halogenated-6-alkylthiotoluene, etc. The effect of high yield and simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
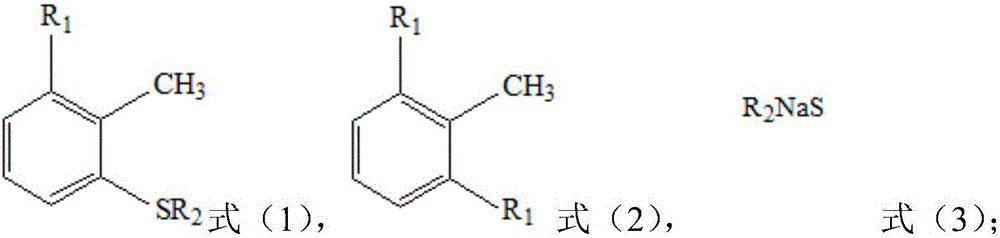
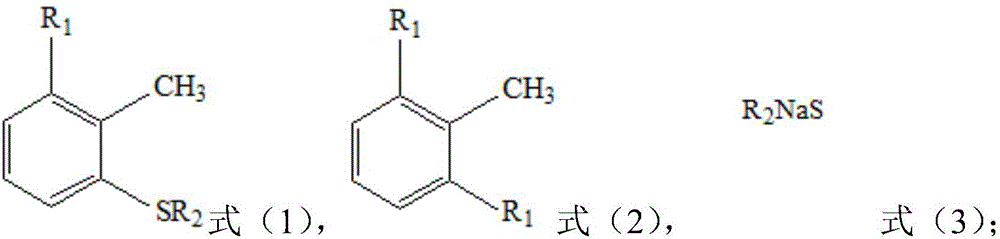
[0014] The present invention provides a kind of preparation method of the 2-halo-6-alkylthiotoluene shown in formula (1), it is characterized in that, this method comprises: the aqueous solution of the compound shown in formula (3) and as The dimethyl sulfoxide of the solvent is mixed, the obtained mixture is dehydrated, and the material obtained after dehydration is contacted with the compound shown in formula (2);
[0015]
[0016] In formula (2), two R 1 Each is F, Cl or Br; preferably, two R 1 Each is Cl or Br; more preferably, two R 1 Both are Cl.
[0017] In formula (3), R 2 for C 1 -C 6 straight-chain or branched-chain alkyl, such as methyl, ethyl, n-propyl, isopropyl, n-butyl, sec-butyl, isobutyl, tert-butyl, n-pentyl, sec-pentyl, 1-ethylpropyl, 2-methylbutyl, tert-amyl, 1,2-dimethylpropyl, isopentyl, neopentyl, n-hexyl, sec-hexyl, tert-hexyl, isohexyl or neohexyl ; preferably, R 2 for C 1 -C 3 Straight-chain alkyl groups, such as methyl, ethyl, n-propyl; ...
Embodiment 1
[0036] This example is used to illustrate the preparation method of 2-chloro-6-methylthiotoluene.
[0037] Add 600g of dimethyl sulfoxide and 633g (1.81mol) of 20% sodium methyl mercaptide aqueous solution into a 2000mL four-necked reaction flask, and carry out water pump negative pressure distillation at 90°C and a vacuum of 2000Pa until there is a small amount of Methyl sulfoxide was distilled off, then 300g (1.81mol) of 2,6-dichlorotoluene was added into a 2000mL four-neck reaction flask, and reacted at 100°C for 3h. After the reaction, the temperature was lowered to 30°C, and 0.18 mol of methyl chloride was passed into the reaction system, and after stirring for 0.5 hours, suction filtration was performed, and the solid obtained by suction filtration was rinsed twice with dimethyl sulfoxide, and the filtrates were combined and carried out. Negative pressure rectification gave 220.6 g (1.27 mol) of 2-chloro-6-methylthiotoluene with a yield of 92.5%.
Embodiment 2
[0039] This example is used to illustrate the preparation method of 2-chloro-6-methylthiotoluene.
[0040]Add 1800g of dimethyl sulfoxide and 1269g (2.72mol) of 15% sodium methyl mercaptide aqueous solution into a 2000mL four-necked reaction flask, and carry out water pump negative pressure distillation at 60°C and a vacuum of 500Pa until there is a small amount of Methyl sulfoxide was distilled off, then 300g (1.81mol) of 2,6-dichlorotoluene was added into a 2000mL four-neck reaction flask, and reacted at 80°C for 1h. After the reaction, the temperature was lowered to 20°C, and 0.091 mol of methyl chloride was introduced into the reaction system. After stirring for 0.5 hours, suction filtration was carried out, and the solid obtained by suction filtration was rinsed twice with dimethyl sulfoxide, and the filtrates were combined and carried out. Negative pressure rectification gave 184.9 g (1.05 mol) of 2-chloro-6-methylthiotoluene with a yield of 93.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com