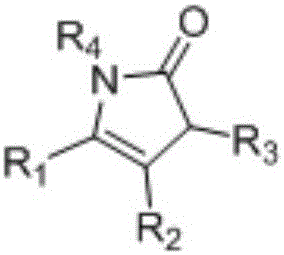
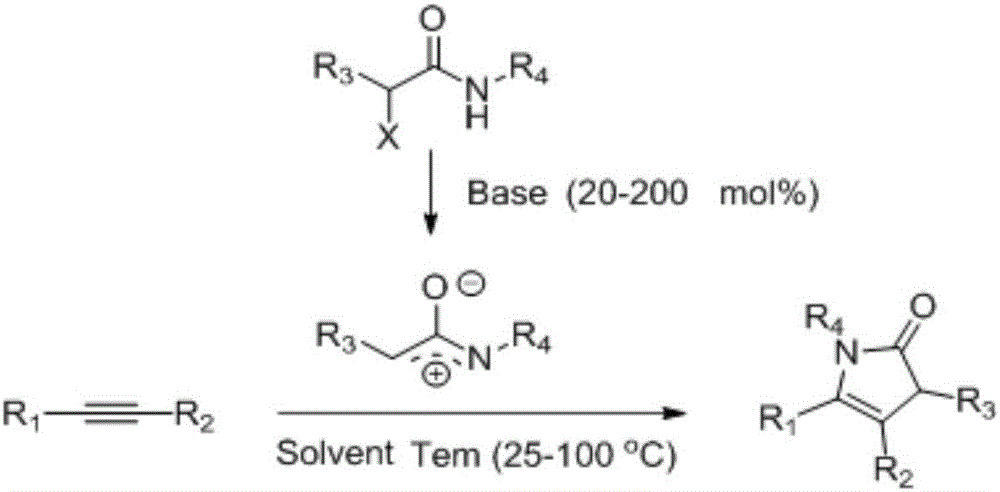
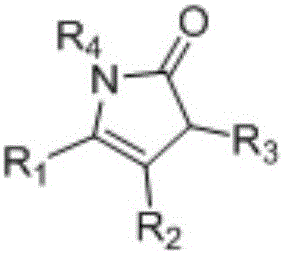
1,3-dihydro-2H-pyrrolidone compounds and synthetic method thereof
A compound, pyrrolidone technology, applied in the field of 1,3-dihydro-2H-pyrrolidone compound and its synthesis, can solve limitations and other problems, achieve high selectivity and yield, and good industrial application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Synthesis of 1-(Benzyloxy)-3,3-dimethyl-5-phenyl-1,3-dihydro-2H-pyrrol-2-one
[0028] Add 0.25 mmol of K to the reaction vessel 2 CO 3 , 0.1mmol phenylacetylene, then add 1ml trifluoromethanol, 0.12mmol α-halogenated amide (N-(benzyloxy)-2-bromo-2-methylpropionamide), react at 25°C, after the reaction, wash with aqueous solution , and then extracted with an organic solvent, dried, concentrated under reduced pressure to remove the solvent, and the crude product was separated by column chromatography to obtain the target product with a yield of 70%.
Embodiment 2
[0030] Synthesis of 1-(benzyloxy)-5-(4-methoxyphenyl)-3,3-dimethyl-1,3-dihydro-2H-pyrrol-2-one
[0031] Add 0.15 mmol of KOH, 0.1 mmol of 4-methoxyphenylacetylene to the reaction vessel, then add 1 ml of trifluoroethanol, 0.11 mmol of α-halogenated amide (N-(benzyloxy)-2-bromo-2-methylpropane Amide) was reacted at 25°C. After the reaction was completed, it was washed with an aqueous solution, then extracted with an organic solvent, dried, and evaporated under reduced pressure to remove the solvent. The crude product was separated by column chromatography to obtain the target product with a yield of 55%.
Embodiment 3
[0033] Synthesis of 1-(benzyloxy)-5-(4-fluorophenyl)-3,3-dimethyl-1,3-dihydro-2H-pyrrol-2-one
[0034] Add 0.15 mmol of NaOH, 0.1 mmol of 4-fluorophenylacetylene to the reaction vessel, then add 1 ml of acetonitrile, 0.12 mmol of α-halogenated amide (N-(benzyloxy)-2-bromo-2-methylpropionamide), 25 ℃ reaction, after the reaction, wash with aqueous solution, then extract with organic solvent, dry, evaporate and concentrate under reduced pressure to remove the solvent, and the crude product is separated by column chromatography to obtain the target product with a yield of 57%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com