Preparation method of Rivaroxaban
A technology of rivaroxaban and aminomethyl, applied in the field of preparation of rivaroxaban, can solve the problems of unsuitable industrial production of rivaroxaban, difficult recovery of solvents, many reaction steps, etc., and achieves easy product separation and reaction The effect of short steps and less waste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
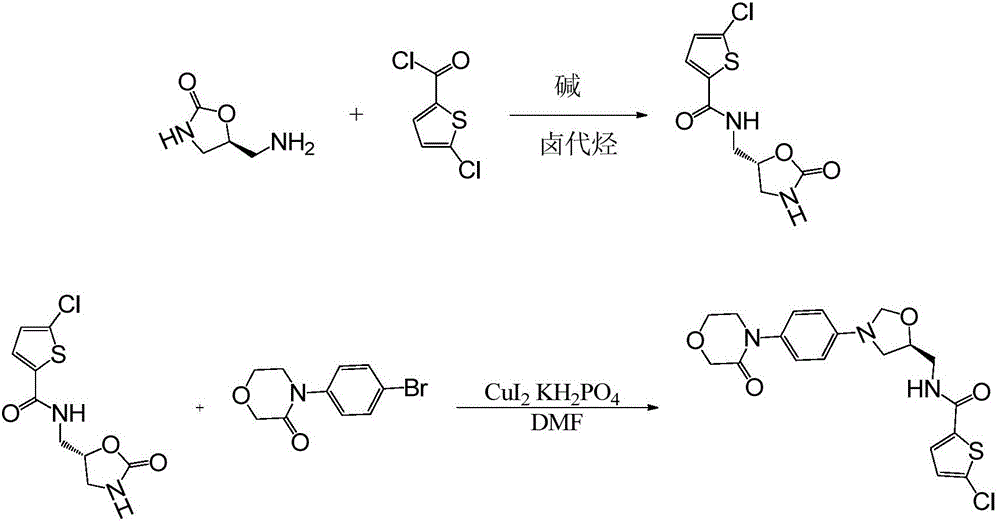
[0027] A preparation method for rivaroxaban, comprising the following steps: in a 2000ml four-neck flask, add solid (s)-5-aminomethyl-2-oxazolidinone 100g (0.86mol), triethylamine 217g (2.15mol), 800g of dichloromethane, slowly dropwise added 202.7g (1.12mol) of 5-chloro-2-acylchlorothiophene. The temperature was raised to 40°C with stirring, and the reaction was completed after reflux for 6 hours. The reaction solution was directly added to ice water, the organic layer was washed with water, dried, and concentrated to obtain an intermediate: 5-chloro-N-((5S)-2-oxo-1,3-oxazolin-5-yl)methyl) Thiophene-2-carboxamide, light yellow solid 181.6g, yield 81%.
[0028] Add 181.6g (0.7mol) of the intermediate obtained in the previous step into a 1000ml four-necked flask, and throw 358g (1.4mol) of 4-(4-bromophenyl)morpholin-3-one and 26.7g of cuprous iodide (0.14mol), potassium dihydrogenphosphate 23.8g (0.175mol) and 1,4-dioxane 910g. Stir and heat up to 105°C to start heat preserv...
Embodiment 2
[0030] A preparation method for rivaroxaban, comprising the following steps: in a 2000ml four-necked flask, add solid (s)-5-aminomethyl-2-oxazolidinone 100g (0.86mol), pyridine 204g (2.58 mol), dichloromethane 800g, and 202.7g (1.12mol) of 5-chloro-2-acylchlorothiophene were slowly added dropwise. The temperature was raised to 40°C with stirring, and the reaction was completed after reflux for 6 hours. The reaction solution was directly added to ice water, the organic layer was washed with water, dried, and concentrated to obtain an intermediate: 5-chloro-N-((5S)-2-oxo-1,3-oxazolin-5-yl)methyl) Thiophene-2-carboxamide, light yellow solid 185g, yield 82.5%.
[0031] 185g (0.71mol) of the intermediate obtained in the previous step was added in a four-necked flask of 1000ml, and 400g (1.56mol) of 4-(4-bromophenyl) morpholin-3-one was cast, and 27.4g (1.56mol) of cuprous iodide ( 0.144mol), potassium dihydrogen phosphate 24g (0.177mol) and DMF910g. Stir and heat up to 110°C to ...
Embodiment 3
[0033] A preparation method for rivaroxaban, comprising the following steps: in a 2000ml four-necked flask, add solid (s)-5-aminomethyl-2-oxazolidinone 100g (0.86mol), potassium carbonate 356g ( 2.58mol), dichloroethane 800g, and 202.7g (1.12mol) of 5-chloro-2-acylchlorothiophene were slowly added dropwise. The temperature was raised to 60°C with stirring, and the reaction was completed after reflux for 6 hours. The reaction solution was directly added to ice water, the organic layer was washed with water, dried, and concentrated to obtain an intermediate: 5-chloro-N-((5S)-2-oxo-1,3-oxazolin-5-yl)methyl) Thiophene-2-carboxamide, light yellow solid 179g, yield 80%.
[0034] 179g (0.69mol) of the intermediate obtained in the previous step was added to a 1000ml four-necked flask, and 441.6g (1.725mol) of 4-(4-bromophenyl)morpholin-3-one and 19.8g of cuprous bromide were cast (0.138mol), dipotassium hydrogen phosphate 39.5g (0.173mol) and DMF910g. Stir and heat up to 110°C to s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com