End-functionalized rubber as well as preparation method and application thereof
A technology of end-group functionalization and rubber, which is applied in the field of preparation of functionalized rubber, and can solve the problems of affecting the rubber modification effect, single function, and reducing the efficiency of end-group functionalization end-capping, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
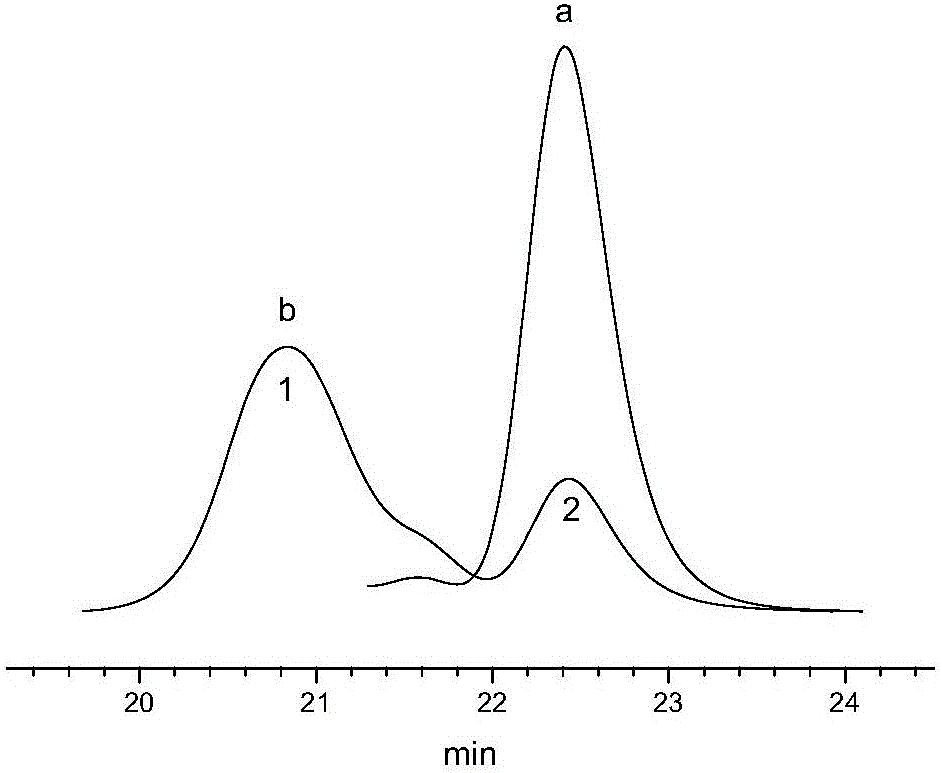
[0076] Synthesize according to the method for comparative example 4, the difference is that the design molecular weight is 100000, after the polymerization reaction, in molar ratio DPE / n-(C 4 h 9 ) Li=1.1, add DPE, react for 30 minutes, and then add end-capping agent TEOS for end-capping. The GPC spectra of SSBR before and after capping are as follows Image 6 shown. The corresponding number-average molecular weights are 103964 and 108365, respectively, and the end-capping efficiency is calculated to be 83.11%.
[0077] Compared with Comparative Example 4, the GPC spectra before and after capping all present a single peak, and the peak shape is basically unchanged (the small peak is caused by oxygen coupling), indicating that after the capping agent DPE is capped, then adding TEOS capped SSBR can Eliminate side reactions such as dimerization.
Embodiment 2
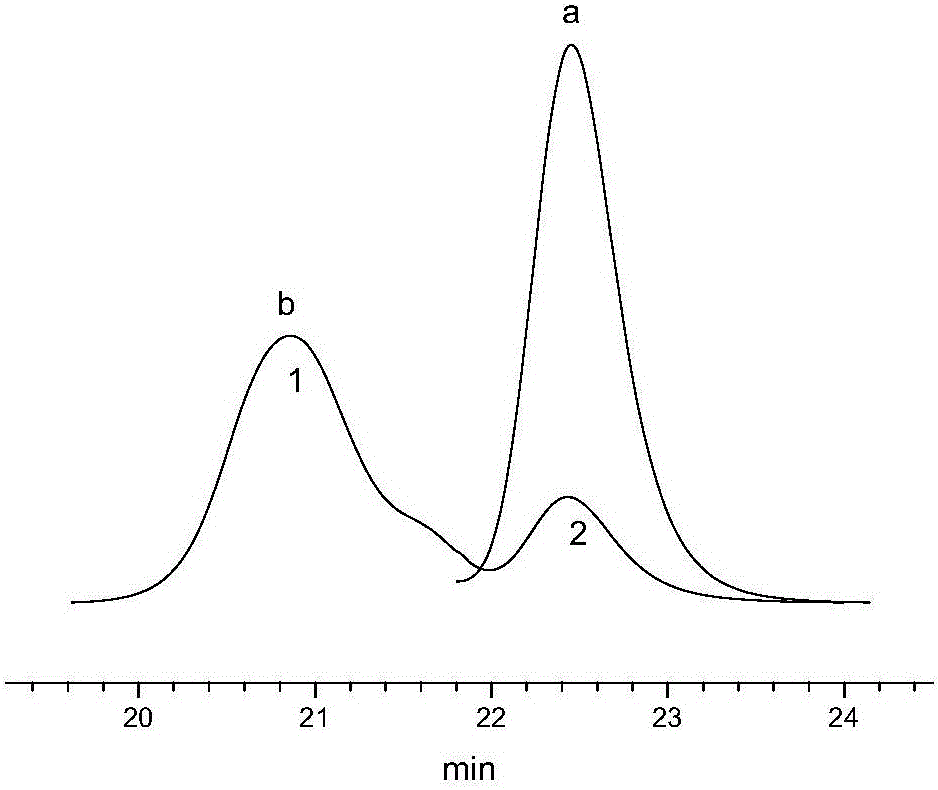
[0079] Synthesize according to the method for comparative example 5, the difference is that the design molecular weight is 100000, after the polymerization reaction, by molar ratio DPE / n-(C 4 h 9 ) Li=1.2, add DPE, react for 30 minutes, and then add end-capping agent DTEOS for end-capping. The GPC spectra of SSBR before and after capping are as follows Figure 7 shown. The corresponding number-average molecular weights are 118322 and 119784, respectively, and the end-capping efficiency is calculated to be 78.9%.
[0080] Compared with Comparative Example 5, the GPC spectra before and after capping all present a single peak, and the peak shape is basically unchanged (the small peak is caused by oxygen coupling). Eliminate side reactions such as dimerization.
Embodiment 3
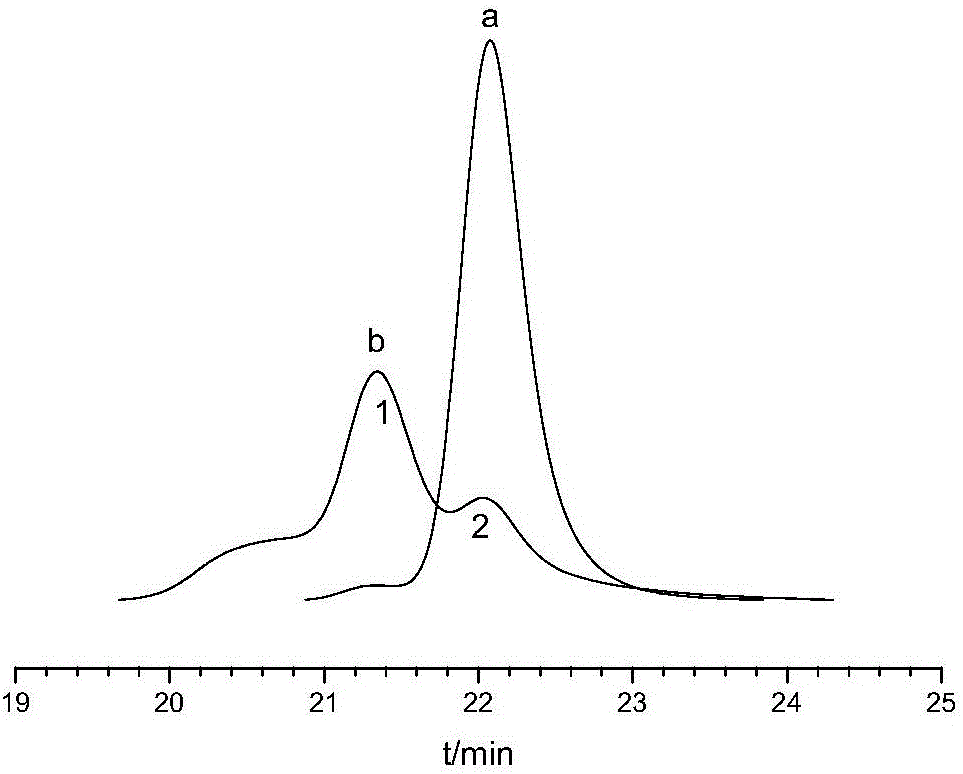
[0082] Synthesize according to the method for comparative example 4, the difference is that the design molecular weight is 100000, after the polymerization reaction, in molar ratio DPE / n-(C 4 h 9 ) Li=1.1 Add DPE, react for 30 minutes, then add end-capping agent GLYME to end-cap. The number-average molecular weights of SSBR before and after capping were 115466 and 118638, respectively, and the capping efficiency was calculated to be 79.6%.
[0083] Similarly, the GPC spectra before and after capping showed a single peak and the peak shape was basically unchanged, indicating that after capping with the capping agent DPE, adding GLYME to cap the SSBR can eliminate side reactions such as dimerization.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com