Preparation method of negative electrode material zinc ferrite
A negative electrode material, zinc ferrite technology, applied in chemical instruments and methods, battery electrodes, iron compounds, etc., can solve problems affecting battery safety performance, lithium precipitation, etc., achieve a wide range of raw material sources, improve safety, and high safety performance Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
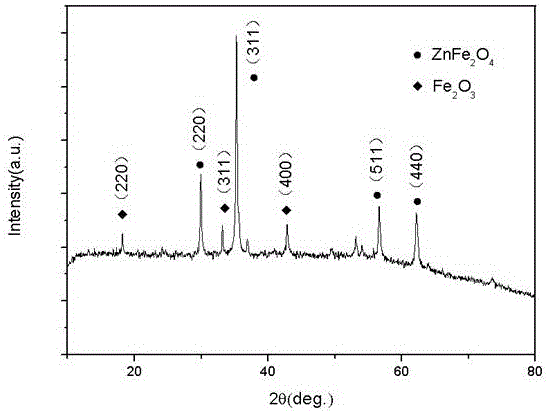
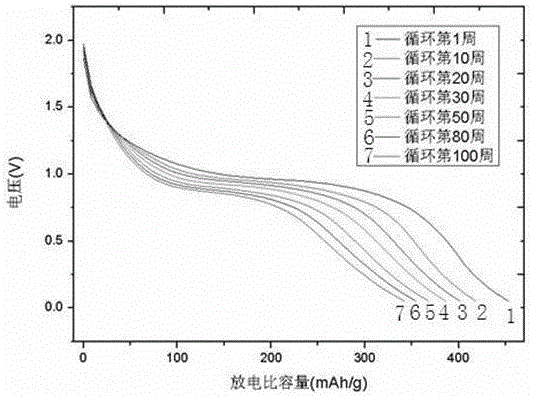
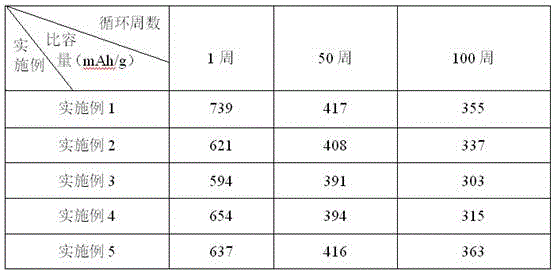
Image
Examples
Embodiment 1
[0025] First, 1.5g polyethylene glycol, 0.028mol ZnCl 2 and 0.059mol FeCl 3 6H 2 Dissolve O in 200ml of deionized water respectively, stir for 0.7h until the solids are completely dissolved, and after ultrasonication for 1h at room temperature, transfer the reaction solution as a whole to a water bath for heating. Add 14.8mol / L ammonia water to adjust the pH of the solution to about 9. After adding ammonia water to adjust the pH, the solution becomes viscous, increase the stirring speed to 500r / min, stop heating after 5 hours of reaction, let the reaction solution stand for 12 hours, and divide the solution After layering, put the sediment in the lower layer into an oven at 100°C to dry and grind, then calcined at 800°C for 5 hours, put the calcined solid into 1mol / L KOH solution, stir, filter, wash, dry, and grind, and finally Calcination was carried out at ℃ for 6 hours to obtain the product zinc ferrite.
Embodiment 2
[0027] First, 1.6g polyethylene glycol, 0.027mol ZnCl 2 and 0.057mol FeCl 3 Dissolve in 300ml of deionized water respectively, stir for 0.7h until the solids are completely dissolved, and after ultrasonication for 1h at room temperature, transfer the reaction solution as a whole to a water bath for heating. Adjust the pH of the solution with 14.8mol / L ammonia water between 8 and 9. After adding ammonia water to adjust the pH, the solution becomes viscous, increase the stirring speed to 500r / min, stop heating after 5 hours of reaction, and let the reaction solution stand for 10 hours. After the solution is layered, put the lower precipitate in an oven at 120°C to dry, grind, and calcinate at 750°C for 5 hours. The calcined solid is put into 1mol / L KOH solution, stirred, filtered, washed, dried, and ground. Calcination was carried out at 900° C. for 8 hours to obtain the product zinc ferrite.
Embodiment 3
[0029] First, 1.3g polyethylene glycol, 0.02mol Zn(NO 3 ) 2 6H 2 O and 0.06mol FeCl 3 6H 2 Dissolve O in 300ml of deionized water respectively, stir for 0.5h until the solids are completely dissolved, and after ultrasonication for 1h at room temperature, transfer the reaction solution as a whole to a water bath for heating. Add 14.8mol / L ammonia water to adjust the pH of the solution to be greater than 9. After adding ammonia water to adjust the pH, the solution becomes viscous, increase the stirring speed to 500r / min, stop heating after 5 hours of reaction, let the reaction solution stand for 11 hours, and the solution is layered Finally, put the lower precipitate in an oven at 100°C to dry and grind, then calcined at 800°C for 5 hours, put the calcined solid into 1mol / L KOH solution, stir, filter, wash, dry, grind, and finally put it in 900°C Calcined for 7h at the lower temperature to obtain the product zinc ferrite.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com