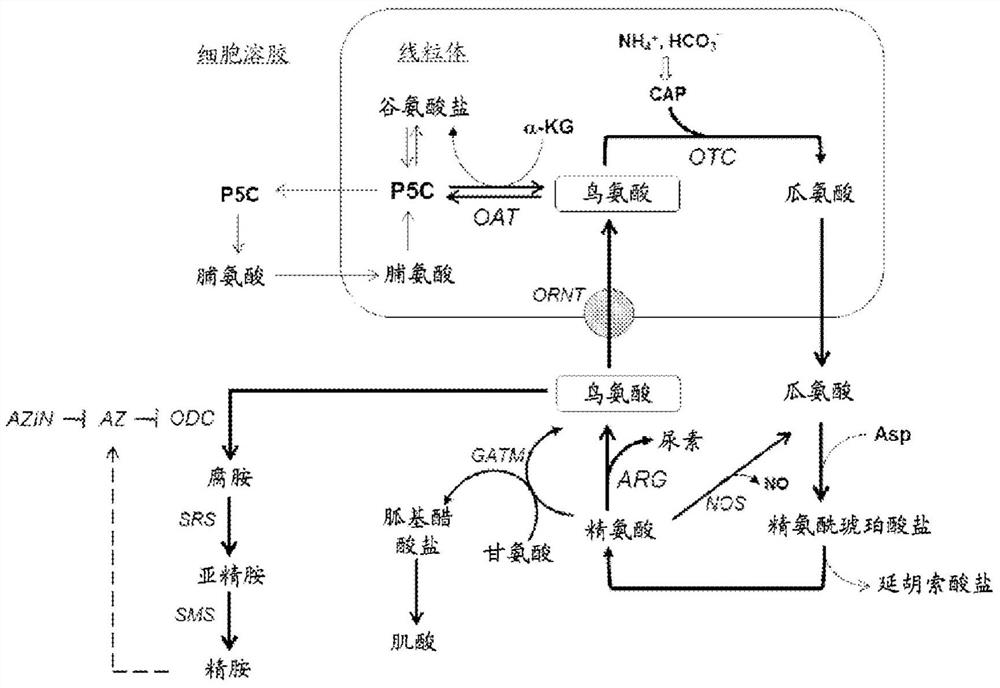
Modulation of ornithine metabolism to manipulate high mannose glycoform content of recombinant proteins
A recombinant protein and high mannose technology, which is applied in immunoglobulin, animal/human protein, tissue culture, etc., can solve problems affecting the therapeutic efficacy of recombinant protein drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
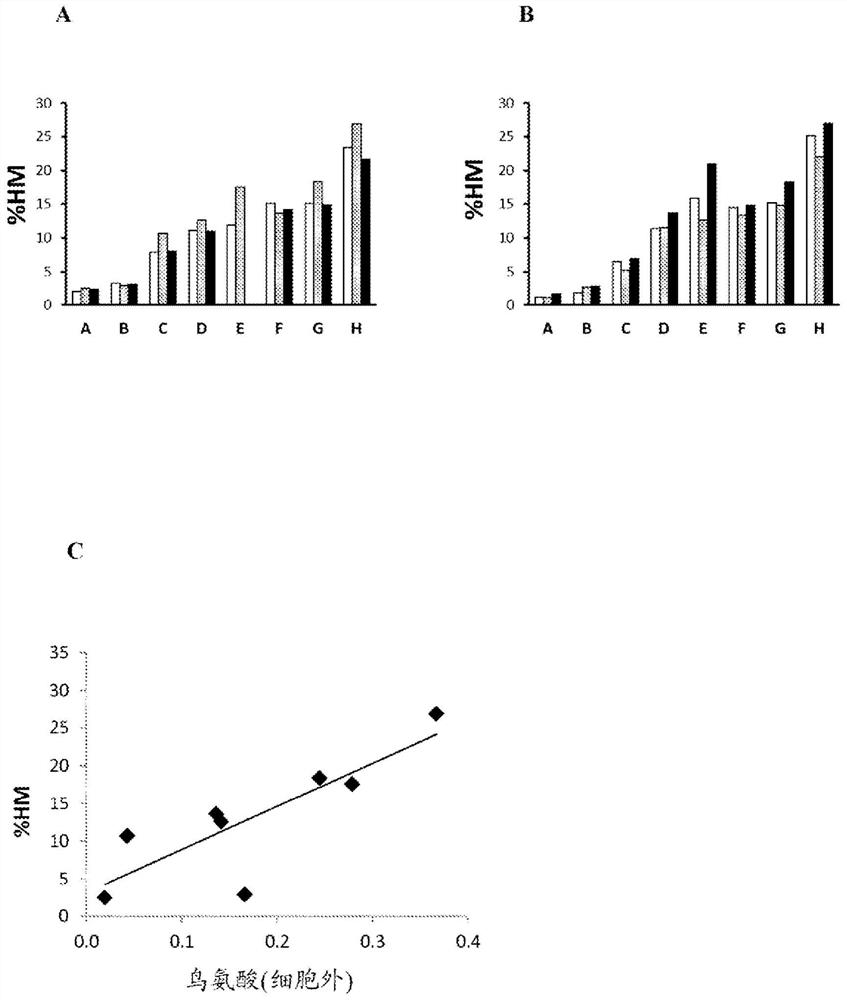
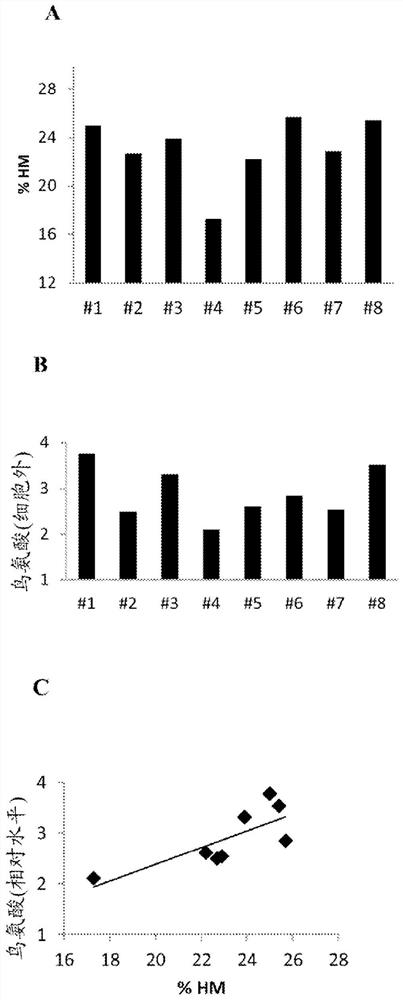
[0123] Extracellular ornithine levels were found to correlate with high mannose glycoform content. Eight CHO cell lines expressing recombinant antibodies with high mannose glycoform content ranging from 20% were selected for this experiment (cell line A-cell line H). Cells were grown in fed-batch culture for 10 days in shake flasks using two different proprietary cell culture media (Medium #1 and Medium #2), each without ornithine. Spent medium samples were removed on days 8, 9, and 10 of culture and subjected to large-scale metabolomics analysis. %HM was determined using the Endo-H rCE-SDS method followed by replacement by the HILIC method described below. Determination of relative levels of ornithine in spent media by large-scale metabolomics analysis in which media components were separated by liquid chromatography and detected by high-resolution spectrometry . Components are identified by matching fragment spectra to a spectral library of known compounds. The relative ...
Embodiment 2
[0131] Arginase 1 mRNA expression levels were determined on selected days during the 10-day fed-batch cultures using the eight cell lines described in Example 1.
[0132] mRNA expression levels were assessed using the QuantiGene Multiplex Assay Kit (Affymetrix, Inc., Santa Clara, CA) according to the manufacturer's instructions.
[0133] Arginase 1 (an enzyme that catalyzes the conversion of arginine to ornithine) was found to be upregulated in a time course-dependent manner in cell lines with higher levels of high mannose, see Figure 4 . This suggests that specific targeting with arginase to block arginase activity and reduce the amount of ornithine produced can be used to reduce high mannan levels.
Embodiment 3
[0135] This example demonstrates that manipulation of the high mannose glycoform content of recombinant glycoproteins is addressed by modulating ornithine accumulation in host cells expressing the recombinant glycoproteins.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com