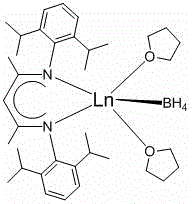
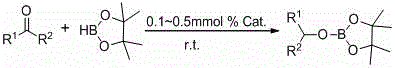Application of beta-diimide bivalent rare earth boron hydrogen complex in catalysis of hydroboration reaction of ketone and boron hydride
A technology of β-diimine-based divalent rare earth and -diimine-based divalent rare earth is applied in the field of rare earth metal complex catalytic reaction, and achieves the effects of wide application range, easy product post-processing and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment one: [2,6- i pr 2 -(C 6 h 3 )-NC(Me)CHC(Me)N-(C 6 h 3 )-2,6- i pr 2 ]Yb-BH 4 . 2THF Catalyzed Hydroboration of Acetophenone and Pinacol Borane
[0025] Add 0.1 mL of catalyst [2,6- i pr 2 -(C 6 h 3 )-NC(Me)CHC(Me)N-(C 6 h 3 )-2,6- i pr 2 ]Yb-BH 4 . 2THF in toluene (0.01 M), then add pinacol borane (0.145 mL, 1 mmol) by syringe, and then add acetophenone (0.117 mL, 1 mmol) by syringe. After reacting for 5 min, add 0.5 mL CDCl 3 , to obtain NMR yield of 90% corresponding pinacol borate, C 6 h 5 CH(CH 3 )OB(OC(CH 3 ) 2 C(CH 3 ) 2 O). NMR data of the product: 1 H NMR (400 MHz, CDCl 3 ): 7.36 (d, 2H, Ar H ),7.16-7.11 (m, 3H, Ar H ), 5.25 (q, 1H, ArC H ), 1.49 (d, 3H, C H 3 CH), 1.21 (d,12H, C(C H 3 ) 2 ) ppm. 11 B NMR (128 MHz, CDCl 3 ): 25.8 ppm.
Embodiment 2
[0026] Embodiment two: [2,6- i pr 2 -(C 6 h 3 )-NC(Me)CHC(Me)N-(C 6 h 3 )-2,6- i pr 2 ]Sm-BH 4 . 2THF Catalyzed Hydroboration of Acetophenone and Pinacol Borane
[0027] Add 0.1 mL of catalyst [2,6- i pr 2 -(C 6 h 3 )-NC(Me)CHC(Me)N-(C 6 h 3 )-2,6- i pr 2 ]Sm-BH 4 . 2THF in toluene (0.01 M), then add pinacol borane (0.145 mL, 1 mmol) with a syringe, then add acetophenone (0.117 mL, 1 mmol) with a syringe, react for 10 min, then add 0.5 mL CDCl 3 , to obtain NMR yield of 92% corresponding pinacol borate, C 6 h 5 CH(CH 3)OB(OC(CH 3 ) 2 C(CH 3 ) 2 O). The NMR data of the product are the same as in Example 1.
Embodiment 3
[0028] Embodiment three: [2,6- i pr 2 -(C 6 h 3 )-NC(Me)CHC(Me)N-(C 6 h 3 )-2,6- i pr 2 ]Sm-BH 4 . 2THF-catalyzed hydroboration of o-methylacetophenone and pinacol-borane
[0029] Add 0.1 mL of catalyst [2,6- i pr 2 -(C 6 h 3 )-NC(Me)CHC(Me)N-(C 6 h 3 )-2,6- i pr 2 ]Sm-BH 4 . 2THF in toluene (0.01 M), then add pinacol borane (0.145 mL, 1 mmol) by syringe, and then add o-methylacetophenone (0.131 mL, 1 mmol) by syringe. After reacting for 10 min, add 0.5 mL CDCl 3 , to obtain NMR yield of 91% corresponding pinacol borate, C 6 h 5 CH(CH 3 )OB(OC(CH 3 ) 2 C(CH 3 ) 2 O). NMR data of the product: 1 H NMR (400 MHz, CDCl 3 ): 7.53 (d, 1H,Ar H ), 7.16 (td, 1H, Ar H ), 7.13 (td, 1H, Ar H ), 7.10 (t, 1H, Ar H ), 5.43 (q,1H, ArC H ), 2.34 (s, 1H, ArC H 3 ), 1.45 (d, 3H, C H 3 CH), 1.21 (d, 12H, C(C H 3 ) 2 ) ppm. 11 B NMR (128 MHz, CDCl 3 ): 25.6 ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com