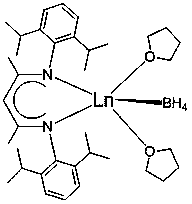
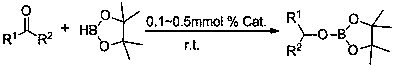Application of β-diimine divalent rare earth borohydride complexes in the catalytic hydroboration of ketones and boranes
A technology of β-diimine divalent rare earth and -diimine divalent rare earth, which is applied in the field of catalytic reaction of rare earth metal complexes to achieve high reaction efficiency, simple and controllable reaction process, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment one: [2,6- i pr 2 -(C 6 h 3 )-NC(Me)CHC(Me)N-(C 6 h 3 )-2,6- i pr 2 ]Yb-BH 4 . 2THF Catalyzed Hydroboration of Acetophenone and Pinacol Borane
[0025] Add 0.1 mL of catalyst [2,6- i pr 2 -(C 6 h 3 )-NC(Me)CHC(Me)N-(C 6 h 3 )-2,6- i pr 2 ]Yb-BH 4 . 2THF in toluene (0.01 M), then add pinacol borane (0.145 mL, 1 mmol) by syringe, and then add acetophenone (0.117 mL, 1 mmol) by syringe. After reacting for 5 min, add 0.5 mL CDCl 3 , to obtain NMR yield of 90% corresponding pinacol borate, C 6 h 5 CH(CH 3 )OB(OC(CH 3 ) 2 C(CH 3 ) 2 O). NMR data of the product: 1 H NMR (400 MHz, CDCl 3 ): 7.36 (d, 2H, Ar H ),7.16-7.11 (m, 3H, Ar H ), 5.25 (q, 1H, ArC H ), 1.49 (d, 3H, C H 3 CH), 1.21 (d,12H, C(C H 3 ) 2 ) ppm. 11 B NMR (128 MHz, CDCl 3 ): 25.8 ppm.
Embodiment 2
[0026] Embodiment two: [2,6- i pr 2 -(C 6 h 3 )-NC(Me)CHC(Me)N-(C 6 h 3 )-2,6- i pr 2 ]Sm-BH 4 . 2THF Catalyzed Hydroboration of Acetophenone and Pinacol Borane
[0027] Add 0.1 mL of catalyst [2,6- i pr 2 -(C 6 h 3 )-NC(Me)CHC(Me)N-(C 6 h 3 )-2,6- i pr 2 ]Sm-BH 4 . 2THF in toluene (0.01 M), then add pinacol borane (0.145 mL, 1 mmol) with a syringe, then add acetophenone (0.117 mL, 1 mmol) with a syringe, react for 10 min, then add 0.5 mL CDCl 3 , to obtain NMR yield of 92% corresponding pinacol borate, C 6 h 5 CH(CH 3)OB(OC(CH 3 ) 2 C(CH 3 ) 2 O). The NMR data of the product are the same as in Example 1.
Embodiment 3
[0028] Embodiment three: [2,6- i pr 2 -(C 6 h 3 )-NC(Me)CHC(Me)N-(C 6 h 3 )-2,6- i pr 2 ]Sm-BH 4 . 2THF-catalyzed hydroboration of o-methylacetophenone and pinacol-borane
[0029] Add 0.1 mL of catalyst [2,6- i pr 2 -(C 6 h 3 )-NC(Me)CHC(Me)N-(C 6 h 3 )-2,6- i pr 2 ]Sm-BH 4 . 2THF in toluene (0.01 M), then add pinacol borane (0.145 mL, 1 mmol) by syringe, and then add o-methylacetophenone (0.131 mL, 1 mmol) by syringe. After reacting for 10 min, add 0.5 mL CDCl 3 , to obtain NMR yield of 91% corresponding pinacol borate, C 6 h 5 CH(CH 3 )OB(OC(CH 3 ) 2 C(CH 3 ) 2 O). NMR data of the product: 1 H NMR (400 MHz, CDCl 3 ): 7.53 (d, 1H,Ar H ), 7.16 (td, 1H, Ar H ), 7.13 (td, 1H, Ar H ), 7.10 (t, 1H, Ar H ), 5.43 (q, 1H,ArC H ), 2.34 (s, 1H, ArC H 3 ), 1.45 (d, 3H, C H 3 CH), 1.21 (d, 12H, C(C H 3 ) 2 ) ppm. 11 B NMR (128 MHz, CDCl 3 ): 25.6 ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com