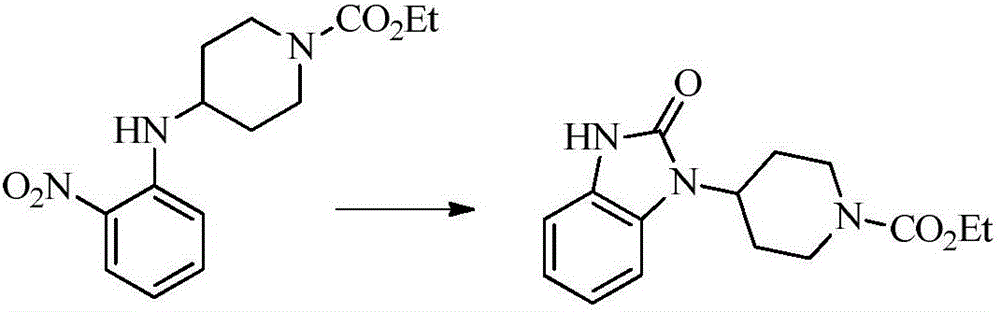
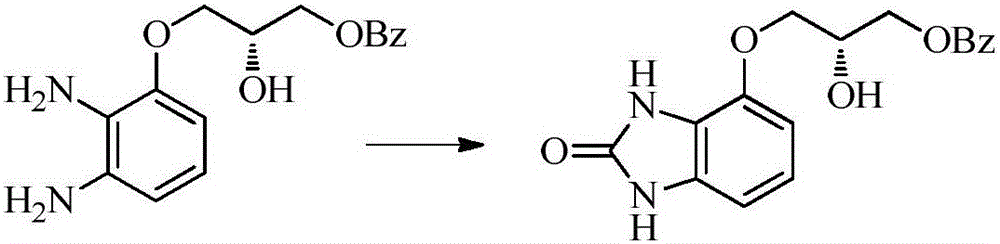
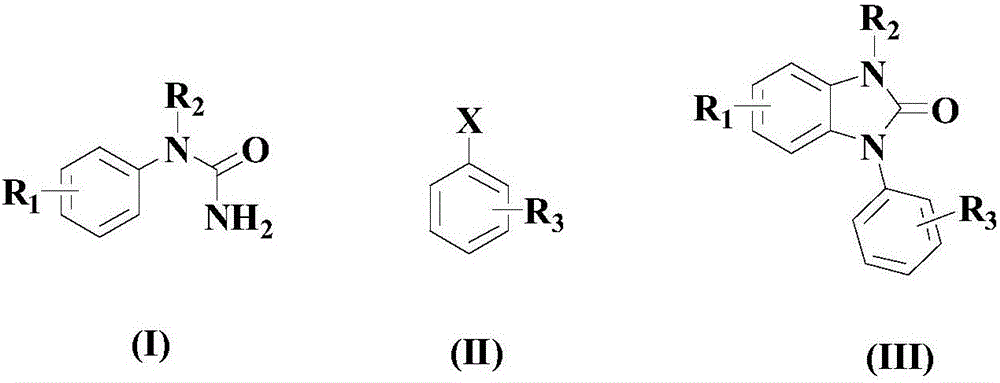
Synthesis method for imidazolone compound
A synthesis method and compound technology, applied in the direction of organic chemistry, etc., can solve the problems such as difficulty in obtaining synthetic starting materials, and the reaction yield needs to be improved, and achieve the effect of broad market prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037]
[0038] At room temperature, in an appropriate amount of organic solvent (a mixture of diethylene glycol monobutyl ether and acetonitrile at a volume ratio of 1:3), add 100 mmol of the above formula (I) compound, 150 mmol of the above formula (II) compound, 10 mmol of catalyst ( It is a mixture of 2.5mmol tris(acetylacetonate)ruthenium and 7.5mmol cerium trifluoromethanesulfonate), 100mmol oxidant cerium ammonium nitrate, 100mmol base tetramethylethylenediamine (TMEDA) and 20mmol auxiliary agent tetraphenylporphyrin; Then the temperature was raised to 70° C., and the reaction was stirred at this temperature for 9 hours;
[0039] After the reaction is over, filter the reaction liquid while it is hot, adjust the pH value of the filtrate to neutral, then fully wash with aqueous sodium carbonate, then add acetone to extract 2-3 times, combine the organic phases, concentrate under reduced pressure, and pass the residue over 200 - 300-mesh silica gel column chromatography...
Embodiment 2
[0042]
[0043] At room temperature, in an appropriate amount of organic solvent (a mixture of diethylene glycol monobutyl ether and acetonitrile at a volume ratio of 1:3), add 100 mmol of the above formula (I) compound, 200 mmol of the above formula (II) compound, 15 mmol of catalyst ( It is a mixture of 3.4mmol tris(acetylacetonate)ruthenium and 11.6mmol cerium trifluoromethanesulfonate), 130mmol oxidant cerium ammonium nitrate, 150mmol alkali tetramethylethylenediamine (TMEDA) and 25mmol auxiliary agent tetraphenylporphyrin; Then the temperature was raised to 80° C., and the reaction was stirred at this temperature for 8 hours;
[0044] After the reaction is over, filter the reaction liquid while it is hot, adjust the pH value of the filtrate to neutral, then fully wash with aqueous sodium carbonate, then add acetone to extract 2-3 times, combine the organic phases, concentrate under reduced pressure, and pass the residue over 200 - 300-mesh silica gel column chromatogra...
Embodiment 3
[0047]
[0048] At room temperature, in an appropriate amount of organic solvent (a mixture of diethylene glycol monobutyl ether and acetonitrile at a volume ratio of 1:3), add 100 mmol of the above formula (I) compound, 250 mmol of the above formula (II) compound, 20 mmol of catalyst ( A mixture of 4mmol tris(acetylacetonate) ruthenium and 16mmol cerium trifluoromethanesulfonate), 160mmol oxidant cerium ammonium nitrate, 200mmol base tetramethylethylenediamine (TMEDA) and 30mmol auxiliary agent tetraphenylporphyrin; to 90°C, and stirred and reacted at this temperature for 6 hours;
[0049] After the reaction is over, filter the reaction liquid while it is hot, adjust the pH value of the filtrate to neutral, then fully wash with aqueous sodium carbonate, then add acetone to extract 2-3 times, combine the organic phases, concentrate under reduced pressure, and pass the residue over 200 - 300-mesh silica gel column chromatography, eluting with a mixture of chloroform and petr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com