Method for synthesizing C2-symmetrical chiral ferrocenyl phosphine compound
A technology for chiral ferrocene and phosphine compounds, applied in chemical instruments and methods, metallocene, organic chemistry, etc., can solve the problems of cumbersome steps, unfavorable for industrial scale-up, low yield, etc. Selective, few-step effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
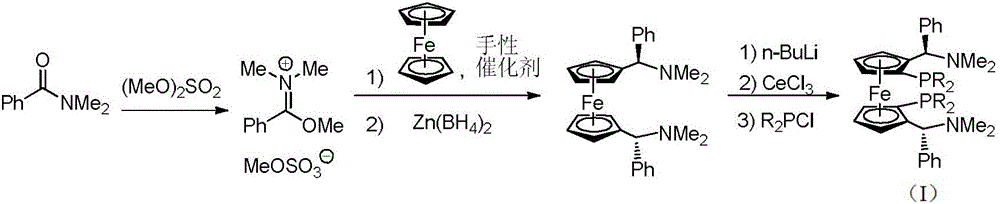
[0021] Embodiment 1 (1) 1, the synthesis of 1 '-bis [(R)-(dimethylamino) (phenyl) methyl] ferrocene
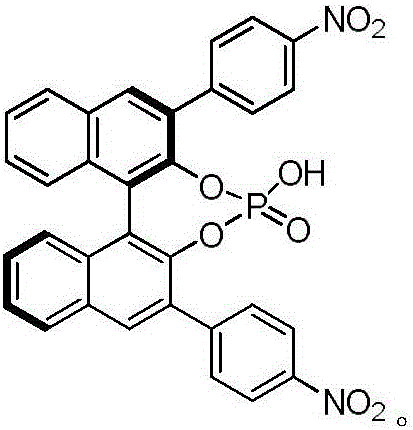
[0022] Add 1L of 1,2-dichloroethane, N,N-dimethylbenzamide (1mol, 149g) and dimethyl sulfate (1mol, 126g) to the dry reactor successively, react at 50°C for 3 hours, Then add ferrocene (0.5mol, 93g) and (R)-3,3'-bis(4-nitrophenyl)-1,1'-binaphthyl phosphonate (0.005mol, 3g), in React at 10°C for 12 hours, then dropwise add 1M zinc borohydride solution in tetrahydrofuran (1mol, 1L), after reacting at 0°C for 2 hours, slowly add 1L of 10% by mass sodium hydroxide aqueous solution dropwise to the system, and then separate the liquids , the organic layer was dried with anhydrous magnesium sulfate, filtered, and the solvent was distilled off under reduced pressure to obtain a yellow solid, which was recrystallized from n-hexane to obtain 1,1'-bis[(R)-(dimethylamino)(phenyl)methyl ] Ferrocene 210g, productive rate 93%, e.e. value 99.3%. 1 H NMR (400MHz, CDCl 3 ),δ:7.42-7.26(m,10H)...
Embodiment 2
[0025] Embodiment 2 (1) 1, the synthesis of 1 '-bis [(R)-(dimethylamino) (phenyl) methyl] ferrocene
[0026] Add 1L 1,2-dichloroethane, N,N-dimethylbenzamide (1.25mol, 186g) and dimethyl sulfate (1.25mol, 157g) to the dry reactor successively, and react at 80°C for 2 hours, then added ferrocene (0.5mol, 93g) and (R)-3,3'-bis(4-nitrophenyl)-1,1'-binaphthyl phosphonate (0.025mol, 14.7g ), react at 30°C for 10 hours, and finally add 1M zinc borohydride solution in tetrahydrofuran (1.5mol, 1.5L) dropwise, and after reacting at 30°C for 1 hour, slowly add 1L of 10% by mass sodium hydroxide dropwise to the system aqueous solution, and then separated, the organic layer was dried with anhydrous magnesium sulfate, filtered, and the solvent was distilled off under reduced pressure to obtain a yellow solid, which was recrystallized from n-hexane to obtain 1,1'-bis[(R)-(dimethylamino)( Phenyl)methyl]ferrocene 215g, yield 95%, e.e. value 99.4%.
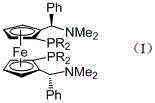
[0027] (2) (S,S)-(-)-2,2'-bis[(R)-(N,N-di...
Embodiment 3
[0029] Embodiment 3 (1) 1, the synthesis of 1 '-bis [(R)-(dimethylamino) (phenyl) methyl] ferrocene
[0030] Add 1L 1,2-dichloroethane, N,N-dimethylbenzamide (1.1mol, 164g) and dimethyl sulfate (1.1mol, 139g) to the dry reactor successively, and react at 70°C for 3 hours, then added ferrocene (0.5mol, 93g) and (R)-3,3'-bis(4-nitrophenyl)-1,1'-binaphthyl phosphonate (0.01mol, 6g) , reacted at 25°C for 12 hours, and finally added dropwise 1M tetrahydrofuran solution of zinc borohydride (1.2mol, 1.2L). After reacting at 25°C for 2 hours, slowly added 1L of 10% by mass aqueous sodium hydroxide solution dropwise to the system , then separated, the organic layer was dried with anhydrous magnesium sulfate, filtered, and the solvent was distilled off under reduced pressure to obtain a yellow solid, which was recrystallized from n-hexane to obtain 1,1'-bis[(R)-(dimethylamino)(benzene Base) methyl] ferrocene 215g, productive rate 95%, e.e. value 99.4%.
[0031] (2) (S,S)-(-)-2,2'-bis[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com