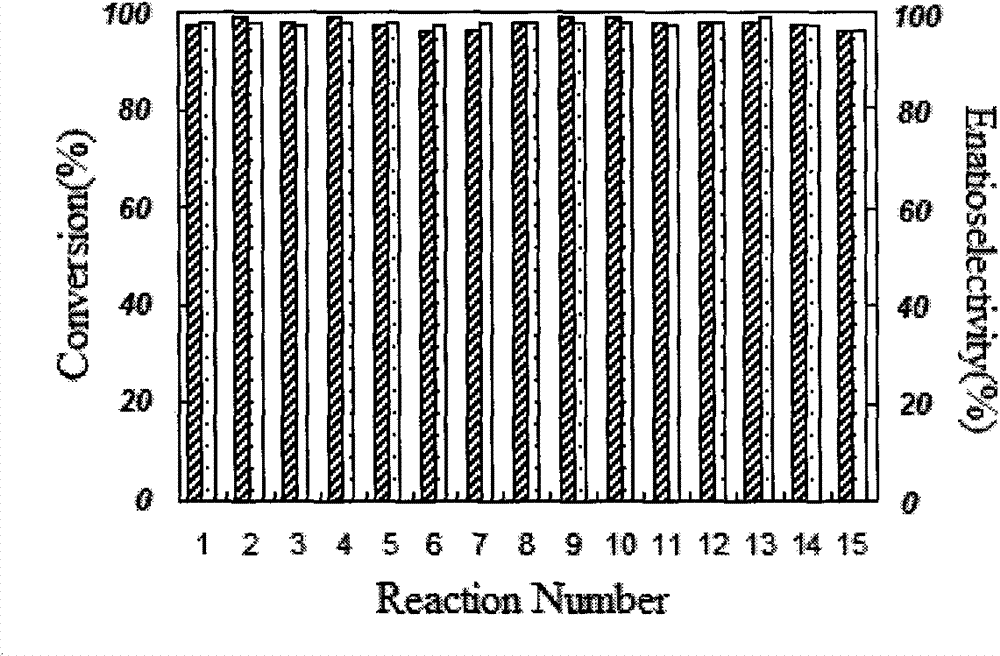
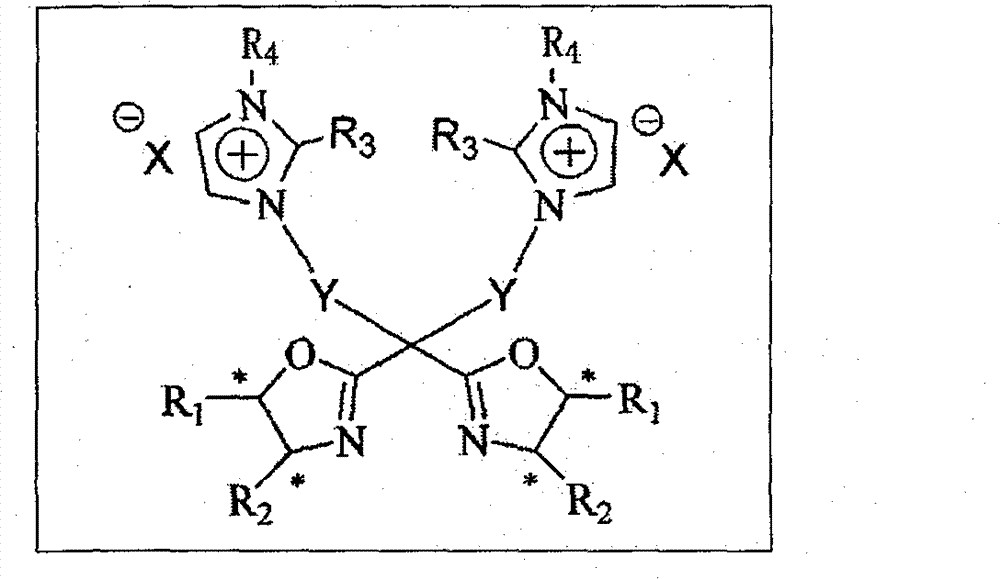
C2 axisymmetric chiral bisoxazoline ligand compound containing imidazole salt ion pair group, and preparation and application thereof
A ligand compound, bisoxazoline technology, applied in the field of chiral bisoxazoline ligands, can solve the problems of unsatisfactory enantioselectivity and recyclability, and achieve easy preparation, good selectivity and good catalysis performance effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] 5,5'-bis[(4S)-4-phenyl-1,3-oxazolin-2-yl]-1,9-bis-(1,2-dimethylimidazole)nonane p-toluenesulfonate Salt synthesis:
[0053] (1) Add 1.00g (3.25mmol) 5,5'-bis[(4S)-4-phenyl-1,3-oxazolin-2-yl]methane in 20ml of anhydrous under the protection of inert gas 4.48ml (2.90M, 2.00eq) n-butyllithium was added dropwise, and reacted for 1h under ice bath, and 1-iodo-4-tert-butyl was slowly added dropwise 3.68g (11.7mmol, 3.60eq) of dimethylsiloxybutane, the temperature was raised naturally after the dropwise addition, and the reaction was carried out for 12h. The reaction was stopped, quenched by adding water dropwise, the reaction solution was extracted 3 times with dichloromethane, the extract was washed 3 times with saturated brine and water respectively, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure, and the oily residue was washed with silica gel Purification by column chromatography (eluent petroleum ether / ethyl acetate 10:1) gav...
Embodiment 2
[0061] 5,5'-bis[(4S)-4-phenyl-1,3-oxazolin-2-yl]-1,9-bis-(1,2-dimethylimidazolium)nonane hexafluorophosphate Synthesis of salt:
[0062] The compound 5,5'-bis[(4S)-4-phenyl-1,3-oxazolin-2-yl]-1,9-bis-(1,2-dimethyl Imidazole)-nonane p-toluenesulfonate (0.292g, 0.300mmol) was dissolved in water (2ml), and a solution of potassium hexafluorophosphate (0.22g, 1.10mmol) in water (2ml) was added, stirred at room temperature for 6h, and the solution A white solid precipitated out in , filtered, washed the filter cake with water, and dried in vacuo to obtain 5,5'-bis[(4S)-4-phenyl-1,3-oxazolin-2-yl]-1,9-di- (1,2-dimethylimidazole)-nonane hexafluorophosphate 0.25 g, yield 86.2%. [α] D 20 =49.3(0.08, CH 3 OH), 1 H NMR (400MHz, DMSO-d 6 ): δ: 7.61~7.57(m, 4H), 7.44~7.26(m, 10H), 5.25~5.22(m, 2H), 4.66(t, J=8Hz, 2H), 4.16~4.07(m, 6H) , 3.73~3.69(m, 6H), 2.65(s, 6H), 2.20~2.08(m, 4H), 1.80~1.76(m, 4H), 1.43~1.41(m, 4H). 13 CNMR (100MHz, DMSO-d 6 ): δ: 169.43, 142.32, 139.31, 128.8...
Embodiment 3
[0064] 5,5′-bis[(4R)-4-tert-butyl-1,3-oxazolin-2-yl]-1,9-bis(1,2-dimethylimidazole)nonane-p-toluenesulfonate Salt preparation
[0065] (1) Replace 5,5'-bis[(4S) in Example 1 with 5,5'-bis[(4R)-4-tert-butyl-1,3-oxazolin-2-yl]methane -4-phenyl-1,3-oxazolin-2-yl] methane, all the other conditions are the same as in Example 1 (1) step, to obtain 5,5'-bis[(4R)-4-tert-butyl- 1,3-oxazolin-2-yl]-1,9-bis(tert-butyl-dimethyl-siloxy)nonane, yield 70.8%. [α] D 20 =-92.3 (c=0.15, MeOH). 1 H NMR (400MHz, CDCl 3 ): δ: 4.11~4.07(dd, J=8.4Hz and 1.6Hz, 2H), 4.02~3.98(dd, J=8.4Hz and 1.6Hz, 2H), 3.86~3.81(t, J=6.4Hz, 2H ), 3.59~3.56(m, 2H), 2.03~2.00(m, 2H), 1.98~1.87(m, 4H), 1.55~1.48(m, 8H), 1.30~1.22(m, 36H), 0.02(s , 18H). 13 C NMR (100MHz, CDCl 3 ): δ: 167.44, 75.62, 68.6, 63.2, 46.2, 34.3, 33.4, 32.8, 26.1, 20.6, 20.7, -5.0. ESI-MS (m / z) = 639.5M+1 + .Anal.Calc.for C 35 h 70 N 2 o 4 Si 2 : C, 65.77; H, 11.04; N, 4.38, Found: C, 65.75; H, 11.10; N, 4.32.
[0066] (2) 5,5'-b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com