Positive electrode of lithium ion secondary battery, and method of manufacturing lithium ion secondary battery
一种二次电池、锂离子的技术,应用在锂离子二次电池的正极和制造锂离子二次电池领域,能够解决锂离子二次电池循环特性不能被提高到足够的值等问题,达到减小锂离子扩散阻力、循环特性提高、提高循环特性的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
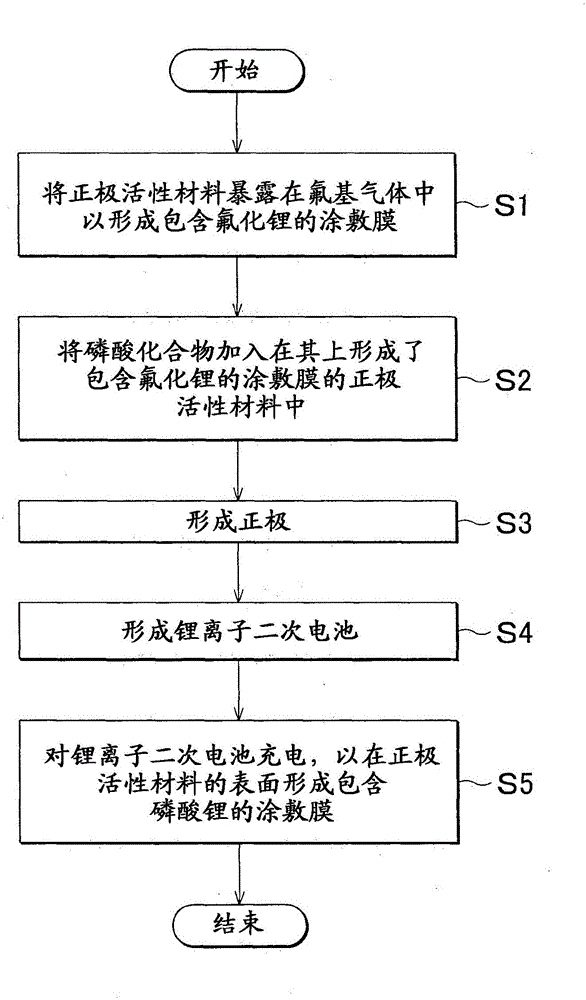
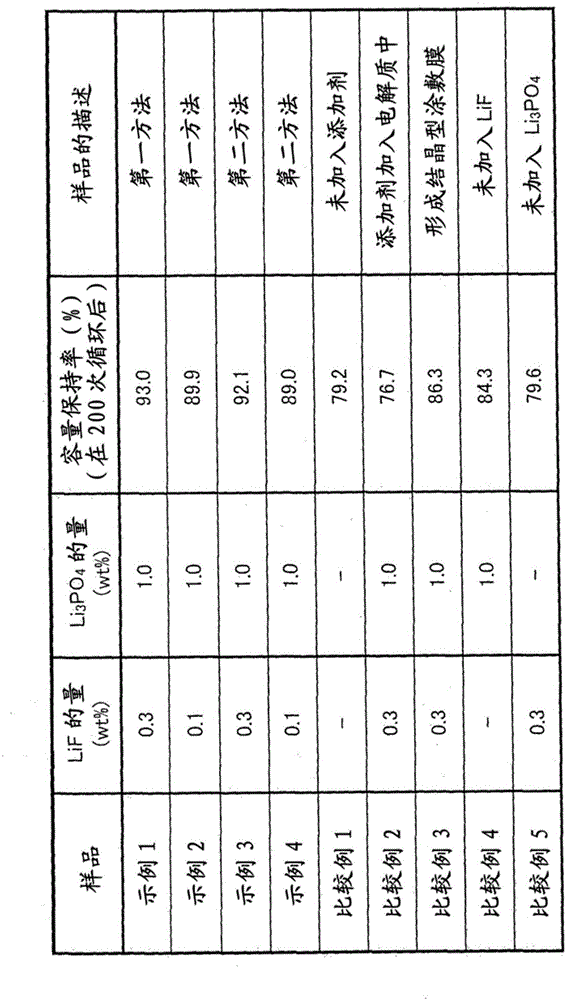
[0067] A lithium ion secondary battery was prepared using the first method described above. As the positive electrode active material, LiNi is used 0.5 mn 1.5 o 4 . In addition, the cathode active material is exposed to fluorine gas in order to fluorinate the surface of the cathode active material. At this time, the fluorination conditions were adjusted so that 0.3 wt% of the LiF coating film relative to the positive electrode active material was formed. The amount of LiF formed on the surface of the cathode active material was measured using X-ray photoelectron spectroscopy (XPS). Below, LiNi on which 0.3wt% LiF is formed 0.5 mn 1.5 o 4 will be referred to as "0.3F-LiNi 0.5 mn 1.5 o 4 ".
[0068] Next, phosphoric acid compound, conductive material and binder are combined with 0.3F-LiNi 0.5 mn 1.5 o 4 mix. Li 3 PO 4 Used as a phosphoric acid compound, acetylene black (AB) as a conductive material, and polyvinylidene fluoride (PVdF) as a binder. At this time, ...
example 2
[0073] As in the case of Example 1, using LiNi 0.5 mn 1.5 o 4 as a positive electrode active material. In addition, the cathode active material is exposed to fluorine gas to fluorinate the surface of the cathode active material. In Example 2, the fluorination conditions were adjusted so that 0.1 wt% of the LiF coating film relative to the positive electrode active material was formed.
[0074] Next, phosphoric acid compound, conductive material and binder are combined with 0.1F-LiNi 0.5 mn 1.5 o 4 mixed. Li 3 PO 4 Used as a phosphoric acid compound, acetylene black (AB) as a conductive material, and polyvinylidene fluoride (PVdF) as a binder. At this time, these components are mixed with each other so that the phosphoric acid compound (Li 3 PO 4 ) is added in an amount of 1 wt%. Specifically, these components are mixed so that their weight ratio (positive electrode active material (0.1F-LiNi 0.5 mn 1.5 o 4 ): phosphoric acid compound (Li 3 PO 4 ): conductive m...
example 3
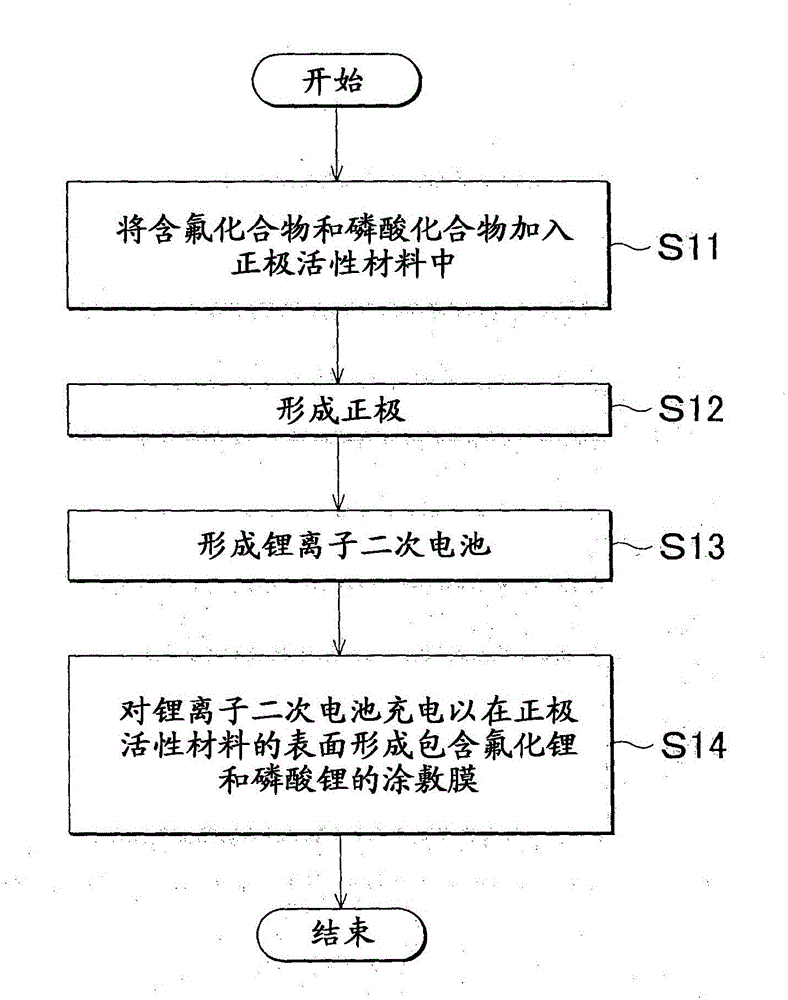
[0076] A lithium ion secondary battery was prepared using the second method described above. First, a positive electrode active material, a fluorine-containing compound, and a phosphoric acid compound are mixed, and further, a conductive material and a binder are mixed with the mixture. LiNi 0.5 mn 1.5 o 4 Used as positive electrode active material, LiF used as fluorine-containing compound, Li 3 PO 4 Used as a phosphoric acid compound, acetylene black (AB) as a conductive material, and polyvinylidene fluoride (PVdF) as a binder. At this time, these components were mixed so that the addition amount of the fluorine-containing compound (LiF) was 0.3 wt%, and the phosphoric acid compound (LiF 3 PO 4 ) is added in an amount of 1 wt%. Specifically, the ingredients are mixed so that their weight ratio (positive electrode active material (LiNi 0.5 mn 1.5 o 4 ): fluorine-containing compound (LiF): phosphoric acid compound (Li 3 PO 4 ): conductive material (AB): binder (PVdF...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com