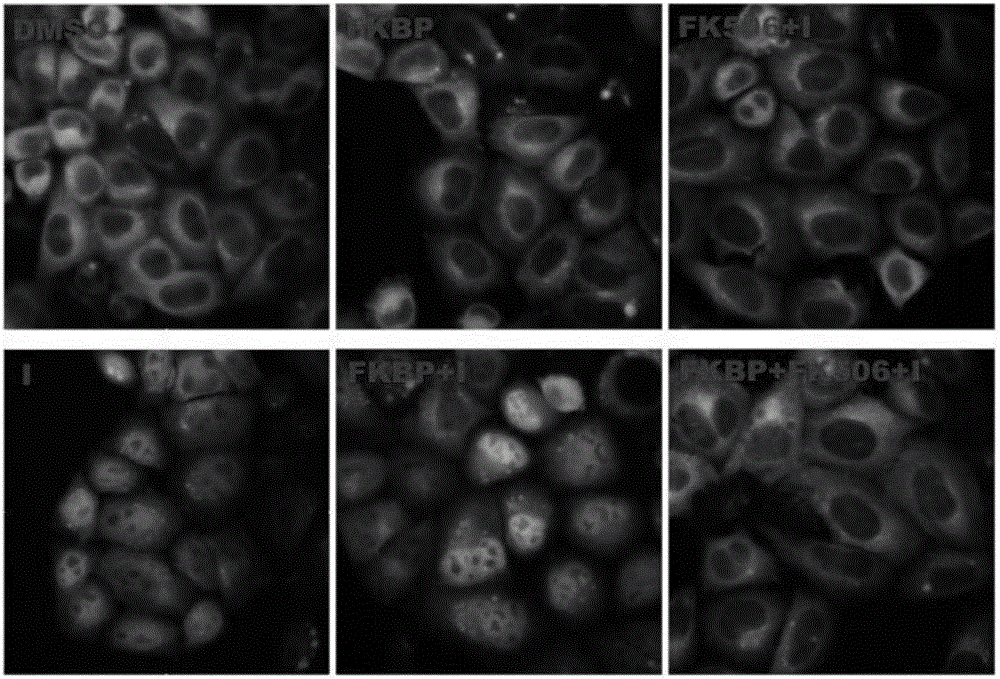
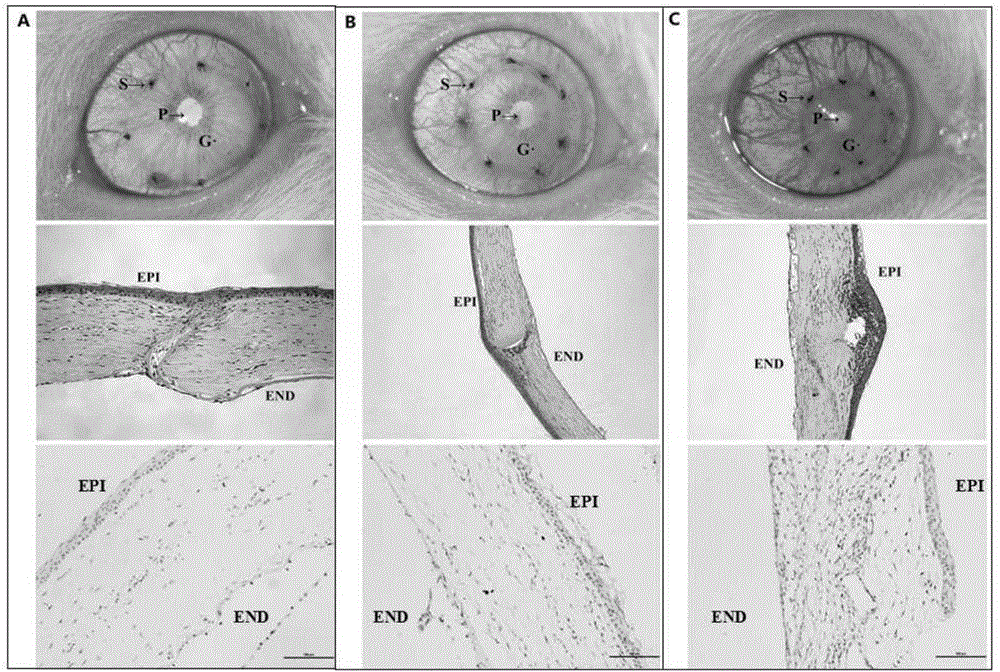
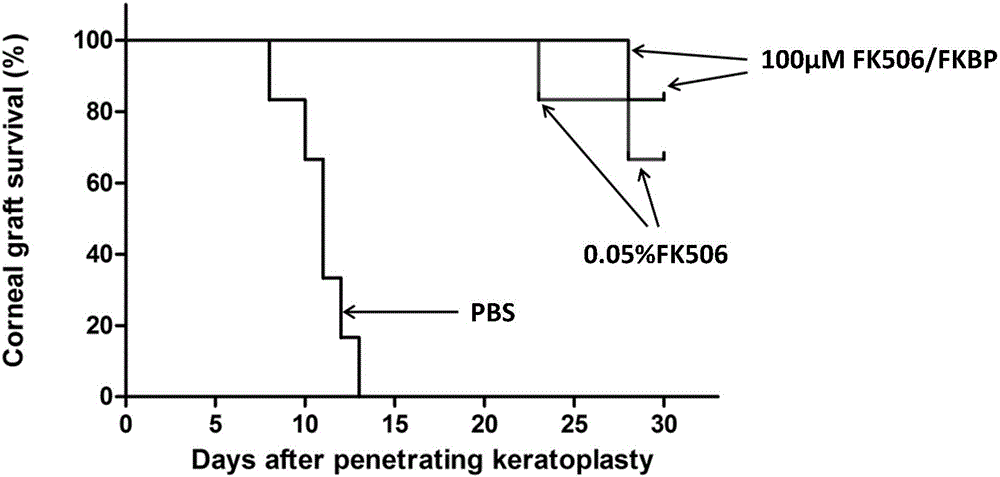
FK506 compound/FKBP protein dimer containing pharmaceutical composition and preparation method thereof
A composition and compound technology, applied in the field of biomedicine, can solve the problems of low solubility, poor stability, easy isomerization, etc., and achieve the effect of improving solubility and poor stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The preparation method of FK506 / hFKBP12a protein dimer aqueous solution is as follows:
[0046] (1) Use an analytical balance to precisely weigh 4 mg of FK506 powder and dissolve it in 0.2 ml of pure DMSO to prepare a DMSO solution of 20 mg / ml (ie about 25 mM) of FK506;
[0047] (2) Dilute the high-purity hFKBP12a protein into a 0.1 mM hFKBP12a protein solution using PBS buffer (osmotic pressure 320 mOsm / L) at pH 7.5;
[0048] (3) Use the hFKBP12a protein solution (50ml) in the above step (2) to slowly dilute the DMSO solution (0.2ml) of FK506 in the step (1), and stir slowly and uniformly to form the FK506 / hFKBP12a protein dimer, thereby 50.2ml of FK506 / hFKBP12a protein dimer aqueous solution was obtained. The molar ratio of FK506 to hFKBP12a protein in the FK506 / hFKBP12a protein dimer aqueous solution is 1:1, and the final concentration of FK506 / hFKBP12a protein dimer is 100 μM. The DMSO solution of 20mg / ml (ie 25mM) of FK506 in step (1) is diluted 250 times with th...
Embodiment 2
[0056] (1) Use an analytical balance to precisely weigh 3.22 mg of FK506 powder and dissolve it in 0.02 ml of absolute ethanol to prepare an ethanol solution of 161 mg / ml (ie about 200 mM) of FK506;
[0057] (2) Dilute the high-purity hFKBP12a protein into a 0.1 mM hFKBP12a protein solution using PBS buffer (osmotic pressure 280 mOsm / L) at pH 7.5;
[0058] (3) Use the hFKBP12a protein solution (40ml) in the above step (2) to slowly dilute the ethanol solution (0.02ml) of FK506 in the step (1), and stir slowly and uniformly to form the FK506 / hFKBP12a protein dimer, thereby 40ml of FK506 / hFKBP12a protein dimer aqueous solution was obtained. The molar ratio of FK506 to hFKBP12a protein in the FK506 / hFKBP12a protein dimer aqueous solution is 1:1, and the final concentration of FK506 / hFKBP12a protein dimer is 100 μM. The ethanol solution of 161mg / ml (i.e. 200mM) of FK506 in step (1) is diluted 2000 times with the 0.1mM hFKBP12a protein solution in step (2), and the organic solvent...
Embodiment 3
[0061] (1) Accurately weigh 3.3 mg of FK506 powder using an analytical balance, and dissolve it in 0.05 ml of pure DMSO to obtain an 80 mM DMSO solution of FK506.
[0062] (2) According to M FK506 :M hFKBP12a蛋白 =1:1.6 ratio, the hFKBP12a protein was dissolved in PBS buffer solution with pH 7.5 to prepare 32ml of 0.2mM hFKBP12a protein solution.
[0063] (3) Mix the solutions obtained in step (1) and step (2) uniformly to form FK506 / hFKBP12a protein dimer, thereby obtaining an aqueous solution of FK506 / hFKBP12a protein dimer (wherein the concentration of FK506 is 0.125mM).
[0064] (4) Then use PBS buffer (pH=7.5, osmotic pressure 260mOsm / L) to dilute 32ml of the FK506 / hFKBP12a protein dimer aqueous solution to 320ml, and then use a 15ml molecular sieve centrifuge tube (centrifuge parameters: 4000g, 40min) The solution was concentrated to 32ml. Thus, an aqueous solution of FK506 / hFKBP12a protein dimer from which the organic solvent DMSO was removed was obtained.
[0065] (5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Osmotic pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com