Application of interleukin 35 to preparation of drugs used for treating autoimmune dermatoses, therapeutic drug, and IL-35-Fc fusion protein
An autoimmune, fusion protein technology, applied in the field of biochemical drugs, can solve the problems of trouble, strong toxicity, easy recurrence, etc., and achieve the effects of inhibiting local infiltration, less toxic and side effects, and easy treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 Preparation of IL-35-Fc plasmid expression vector and expression and purification of IL-35-Fc protein
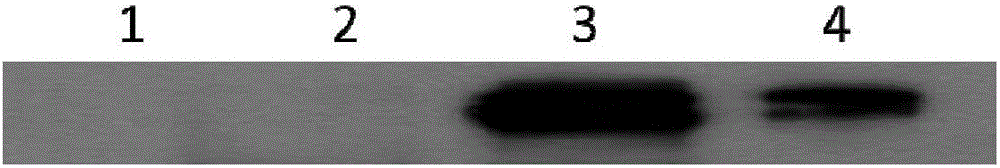
[0042] Construction of plasmid expression vector: pcDNA3.1 containing IL-35-Fc target gene sequence (coding sequence with signal peptide, its nucleotide sequence is shown in SEQ ID NO.6) synthesized by Nanjing GenScript -IL-35-Fc plasmid, using PCR to amplify the full-length sequence of the IL-35-Fc gene from the pcDNA3.1-IL-35-Fc plasmid, the amplified full-length sequence of the IL-35 gene is the same as that of pcDNA 3.4 TA cloning vectors were connected and transformed into competent bacteria. Colony PCR identification was performed on the grown clones, and then the positive clones identified by PCR were sequenced and analyzed. The correct comparison was the successful construction of recombinant IL-35- Fc expression plasmid pcDNA 3.4-IL35-Fc.
[0043] The expression plasmid pcDNA3.4-IL-35-Fc was transfected into suspension-cultured Expi293F cells (pur...
Embodiment 2
[0045] Example 2 Results of animal experiment evaluation of psoriasis mice treated with IL-35-Fc fusion protein of the present invention
[0046] Seven K14-VEGF transgenic mice (purchased from Jackson Lab (Bar Harbor, ME, USA)) (10 weeks old) in each group were divided into 2 groups, according to the expression kinetics of IL-35-Fc fusion protein in mice , we treated the mice once every other day, 10 times in total, 5 μg / mouse / time (pre-experimental injection doses were 2.5 μg / mouse / time, 5 μg / mouse / time and 10 μg / mouse / time, and finally Determine the optimal therapeutic dose is 5μg / only / time). After the treatment, the ear thickness of the mice was measured, and the ears of the mice were taken to weigh the ears, and the ear tissues of the mice were scored according to the PISA scoring system, including redness, scales, and erythema. The ear tissues were taken for H&E staining, and the ear tissues were scored according to the Baker scoring standard. This treatment experiment ...
Embodiment 3
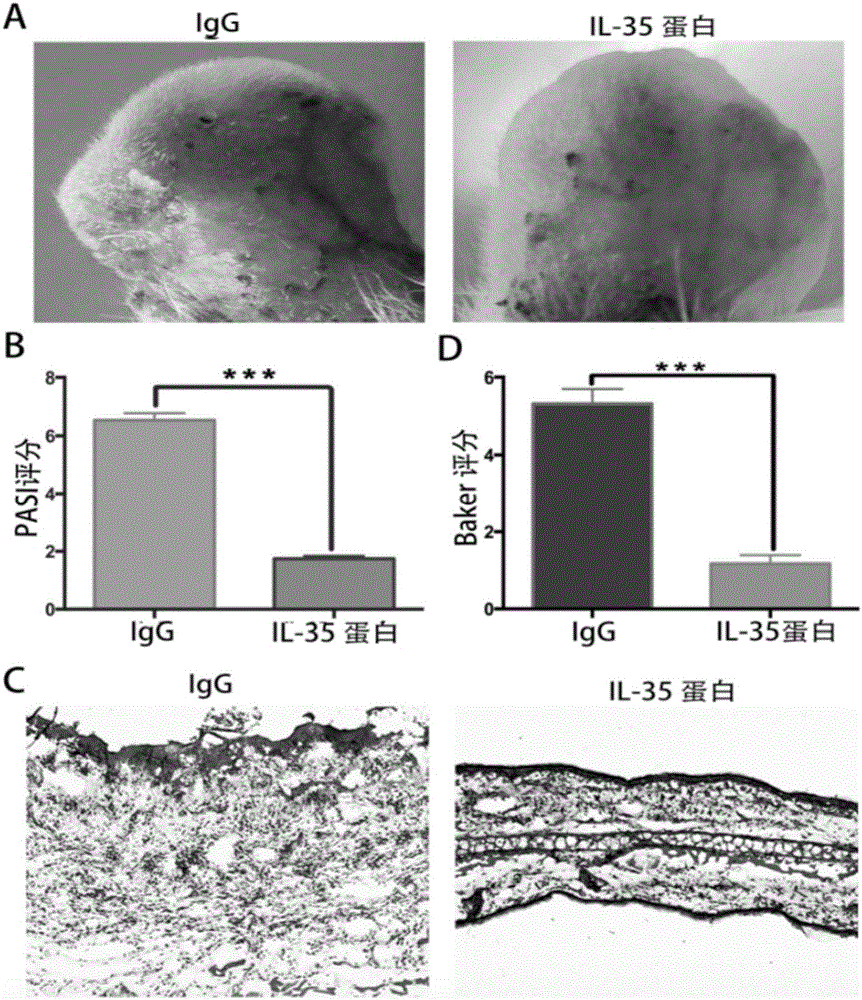
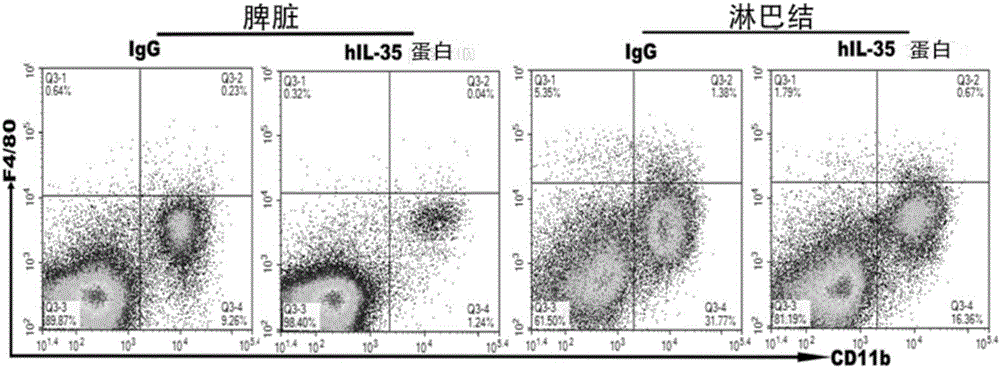
[0050] Example 3 The animal experiment evaluation results of the IL-35-Fc fusion protein of the present invention in the treatment of imiquimod IMQ-induced psoriasis mice.
[0051] The purchased Babl / c mice were divided into two groups for treatment: IgG group, 8 mice; IL-35-Fc fusion protein (5 μg), 8 mice (IL-35 group shown in the figure); after a total of seven treatments Do model building. The back hair of the mice was shaved clean with a razor to expose the back skin, and then the back skin was smeared with 5% imiquimod cream (IMQ) (62.5 mg / mouse / time). Apply it continuously for 6 days, and observe the morbidity of mice of various races every day. During the modeling process, the above-mentioned treatment plan was continued for three times, and the skin tissue of the mice was collected on the second day after the last treatment. Skin tissue was used for histopathological examination, and H&E staining was used to evaluate the severity of psoriasis lesions. The results s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com