Imidazopyridine rhodamine salicylaldehyde copper ion ratio fluorescence probe and application thereof
A ratiometric fluorescent probe, clear salicylaldehyde technology, applied in fluorescence/phosphorescence, luminescent materials, material analysis by optical means, etc., can solve the problem of less ratiometric fluorescent probes, and achieve strong resistance to other ions interference , Unique fluorescence selectivity, high sensitivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015]
[0016] The specific synthesis steps are as follows:
[0017] Add 0.704g (1.0 mmol) imidazo[1,5-a]pyridine rhodamine hydrazide, 0.122g (1.0 mmol) salicylaldehyde, 20 mL absolute ethanol in sequence to a 50 mL round-bottomed flask, reflux at 80 degrees React for 3-6 hours. After TLC detected the reaction, it was concentrated under reduced pressure, and 0.707 g of off-white solid was obtained by column chromatography with a yield of 89.0%.
[0018] H NMR spectrometry: 1 H NMR (400 MHz, CDCl 3 ): δ11.03(s, 1H), 8.43(s, 1H), 7.95(m, 1H), 7.72 (dd, J = 4.0 Hz, 8.0 Hz, 1H), 7.55-7.41 (m, 5H), 7.10-7.01 (m,3H), 6.70-6.67 (m, 2H), 6.56 (m, 2H), 6.46 (d, J = 12.0 Hz, 1H), 6.42 (d, J =4.0 Hz, 1H), 6.31 (dd, J = 2.8 Hz, 8.0 Hz, 1H), 3.82 (s, 4H), 3.34 (q, J =8.0 Hz, 4H), 3.28 (s, 4H), 2.96 (d, J = 4.0 Hz, 4H), 1.82 (m, 2H), 1.62 (s, 2H), 1.44 (m, 2H), 1.17 (t, J = 8.0 Hz, 6H), 0.97 (t, J = 8.0 Hz, 3H).
Embodiment 2
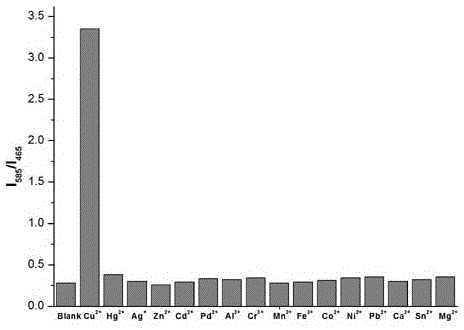
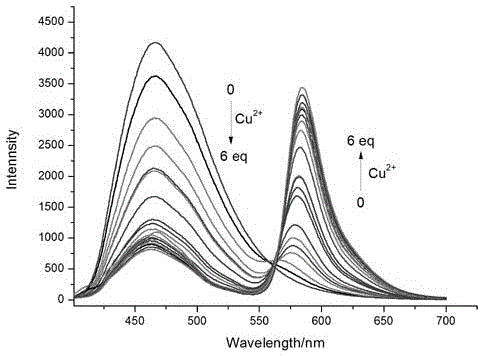
[0020] To the compound of formula (1) (10 -5 M) were added 2 equivalents of Mg to the ethanol solution 2+ , Ca 2+ , Al 3+ , Sn 2+ , Pb 2+ ,Cr 3+ , Mn 2+ , Fe 3+ , Co 2+ , Ni 2+ , Zn 2+ , Cd 2+ ,Pd 2+ ,Hg 2+ and Ag + After measuring the change in the ratio of the fluorescence emission intensity at 465nm and 585nm, it was found that the compound of formula (1) has a strong effect on Cu 2+ Unique fluorescence selectivity, adding 1 equivalent of Cu 2+ After , the fluorescence intensity of compound 1 at 465 nm was significantly reduced, and at the same time, the fluorescence intensity at 585 nm was significantly enhanced. 585 / I 465 = 3.35, as figure 2 shown.
Embodiment 3
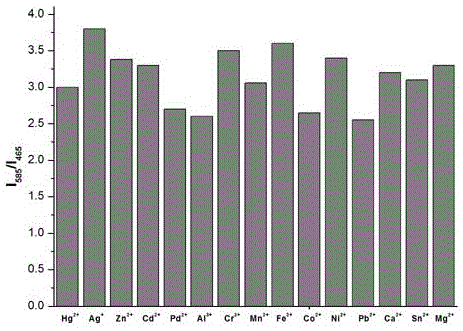
[0022] Compounds of formula (1) (10 -5 M) and 1 equivalent of Cu 2+ 2 equivalents of Mg were added to the ethanol solution 2+ , Ca 2+ , Al 3+ , Sn 2+ , Pb 2+ ,Cr 3+ , Mn 2+ , Fe 3+ , Co 2+ , Ni 2+ , Zn 2+ , Cd 2+ ,Pd 2+ ,Hg 2+ and Ag + After measuring the change of the ratio of fluorescence emission intensity at 465nm and 585nm, it is found that the compound of formula (1) has strong anti-interference ability to other ions, such as image 3 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com