Time-resolved fluorescence immunochromatographic test strip for detecting vancomycin and preparation method and application thereof
A time-resolved fluorescence and vancomycin technology, which is applied in testing pharmaceutical preparations, analytical materials, biological testing, etc., can solve problems such as being unsuitable for large-scale clinical promotion, high cost, and inability to meet clinical monitoring needs, reducing costs. The effect of bottom fluorescence intensity, improving stability and eliminating interference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
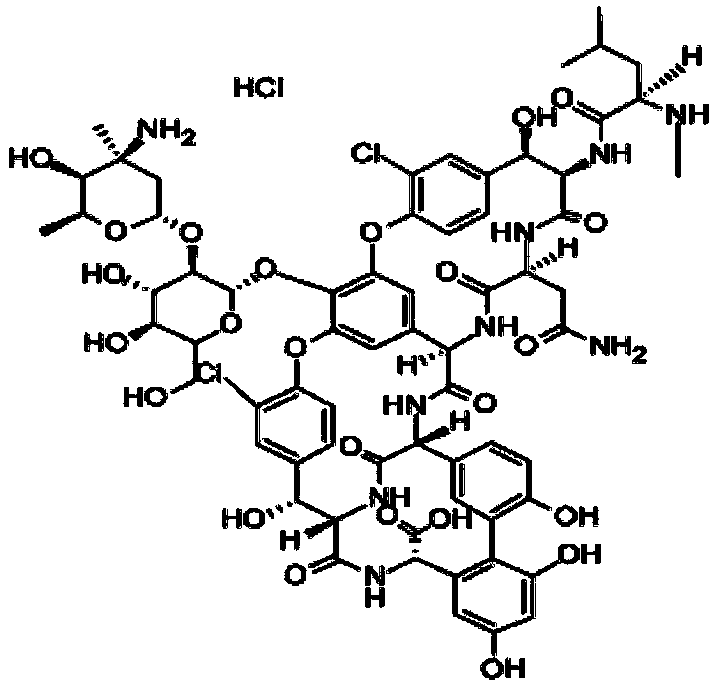
[0026] The invention provides a kind of preparation method of vancomycin-bovine serum albumin conjugate, comprising the following steps:
[0027] 1) mixing an aqueous solution of sodium periodate and an aqueous solution of vancomycin to perform a redox reaction to obtain a vancomycin pre-conjugate;
[0028] 2) mixing bovine serum albumin with a carbonate buffer to obtain a bovine serum albumin solution;
[0029] 3) mixing the vancomycin pre-conjugate described in step 1) with the bovine serum albumin solution described in step 2), and performing a coupling reaction to obtain a vancomycin-bovine serum albumin conjugate;
[0030] The steps 1) and 2) are not limited in time sequence.
[0031] In the present invention, the reason for the preparation of vancomycin-bovine serum albumin conjugates is that vancomycin is a small molecular substance, which has only immunoreactivity but no immunogenicity, and cannot induce the body to produce an immune response. Therefore, vancomycin T...
Embodiment 1
[0091] Example 1 A preparation method of a time-resolved fluorescent immunochromatographic test strip for detecting vancomycin
[0092] 1. Synthesis and identification of vancomycin hapten-carrier protein conjugates
[0093] (1) Preparation of vancomycin-bovine serum albumin conjugate:
[0094] 10 mg / mL NaIO in 1 mL 4 To the solution, 0.5 mL of ultrapure water in which 5 mg of vancomycin was dissolved was added, and stirred at room temperature in the dark for 1 h. The reaction mixture was added to 1mL of 5mg / mL bovine serum albumin solution (50mmol / L carbonate buffer, pH9.6), with 1mol / L Na 2 CO 3 The solution was adjusted to a pH of about 9, stirred for 12 hours, dialyzed against water for 5 times, and stored at -70°C for later use.
[0095] (2) Preparation of vancomycin-chicken ovalbumin conjugate:
[0096] Dissolve 10mg of chicken ovalbumin in 2mL of 0.1M pH7.4 phosphate buffer; add 10mg of vancomycin to the above solution and stir evenly, then add 3mg of 1-ethyl-3-(-3...
Embodiment 4
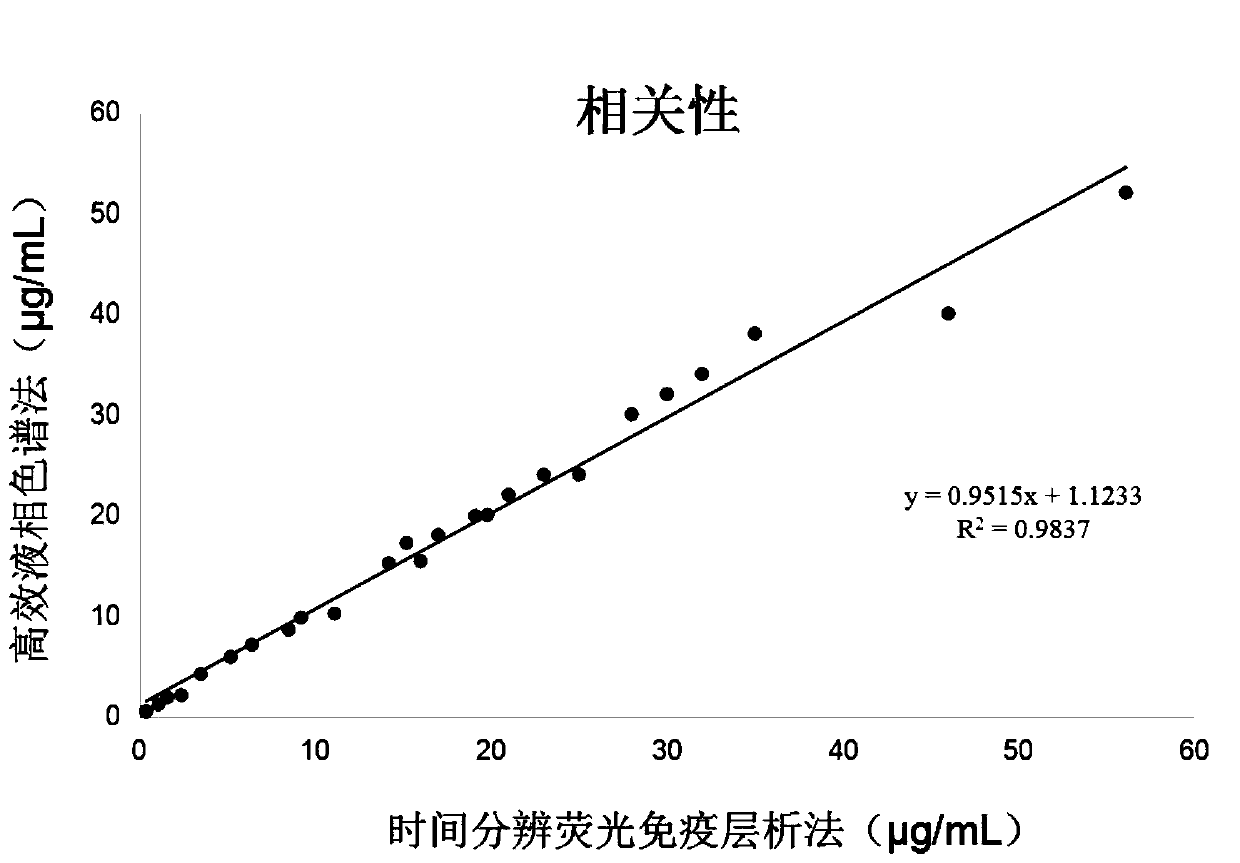
[0132] Embodiment 4 Correlation Analysis of Test Strip Measurement Results of Embodiment 1 of the present invention
[0133] The test strip of Example 1 of the present invention is compared with the method for determining vancomycin in human serum by high performance liquid chromatography, wherein the human serum is a clinical serum sample. High performance liquid chromatography according to the following scheme: adopt Waters 2695 type high performance liquid chromatography, C18 column (150mm * 4.6mm, 5 μ m), mobile phase: the 50mmol / L potassium dihydrogen phosphate buffer solution of pH3.2-acetonitrile (90 : 10); detection wavelength: 230nm; flow rate: 1mL / min; 400 μl of serum samples were added with 50 μl of 10% zinc sulfate to precipitate protein, after centrifugation at 5000r / min, 20 μl of samples were taken to measure the peak height of vancomycin. The results are shown in Table 2. Correlation curve see figure 1 , it can be seen that the two fit well.
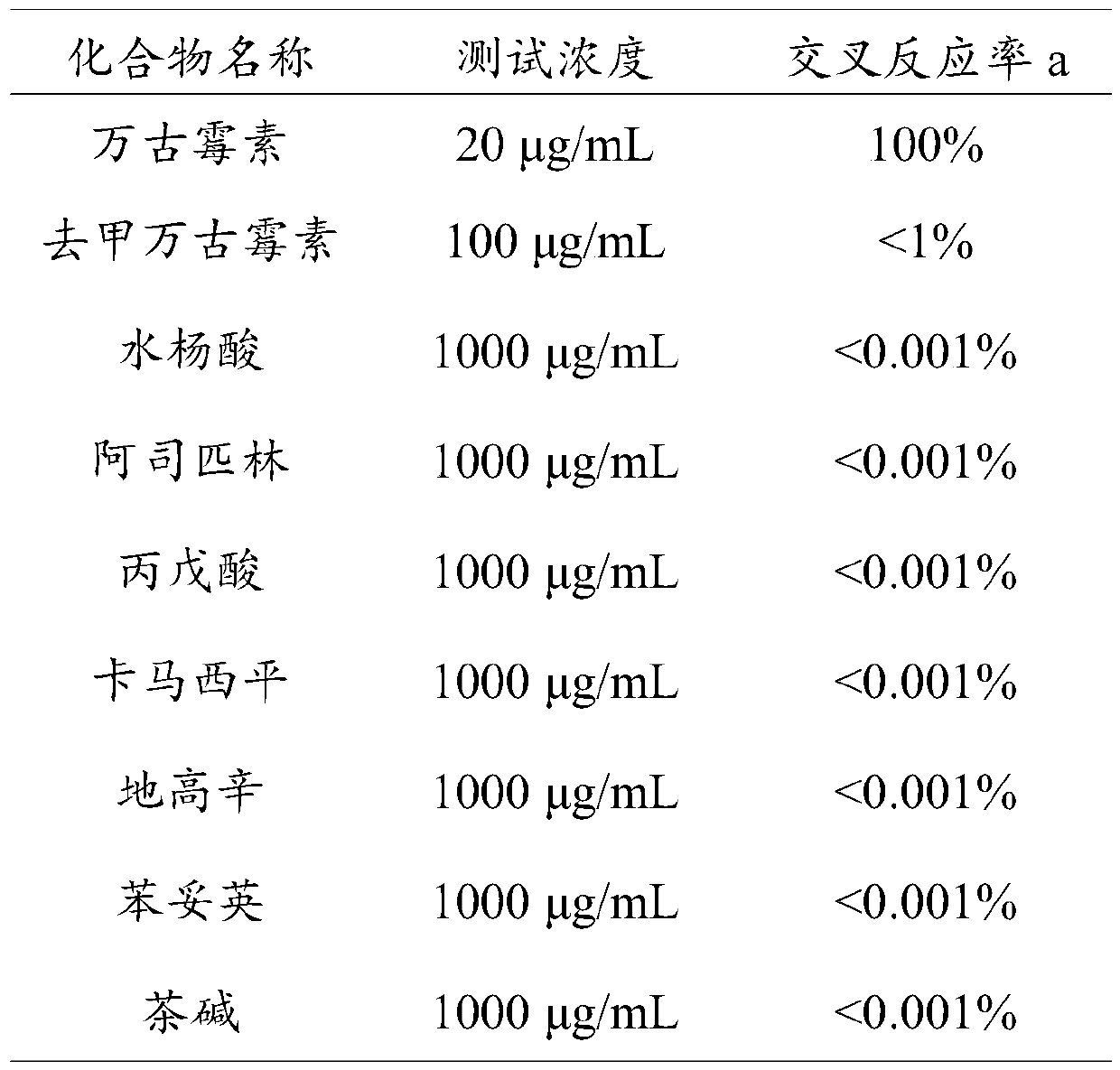
[0134] The test...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com