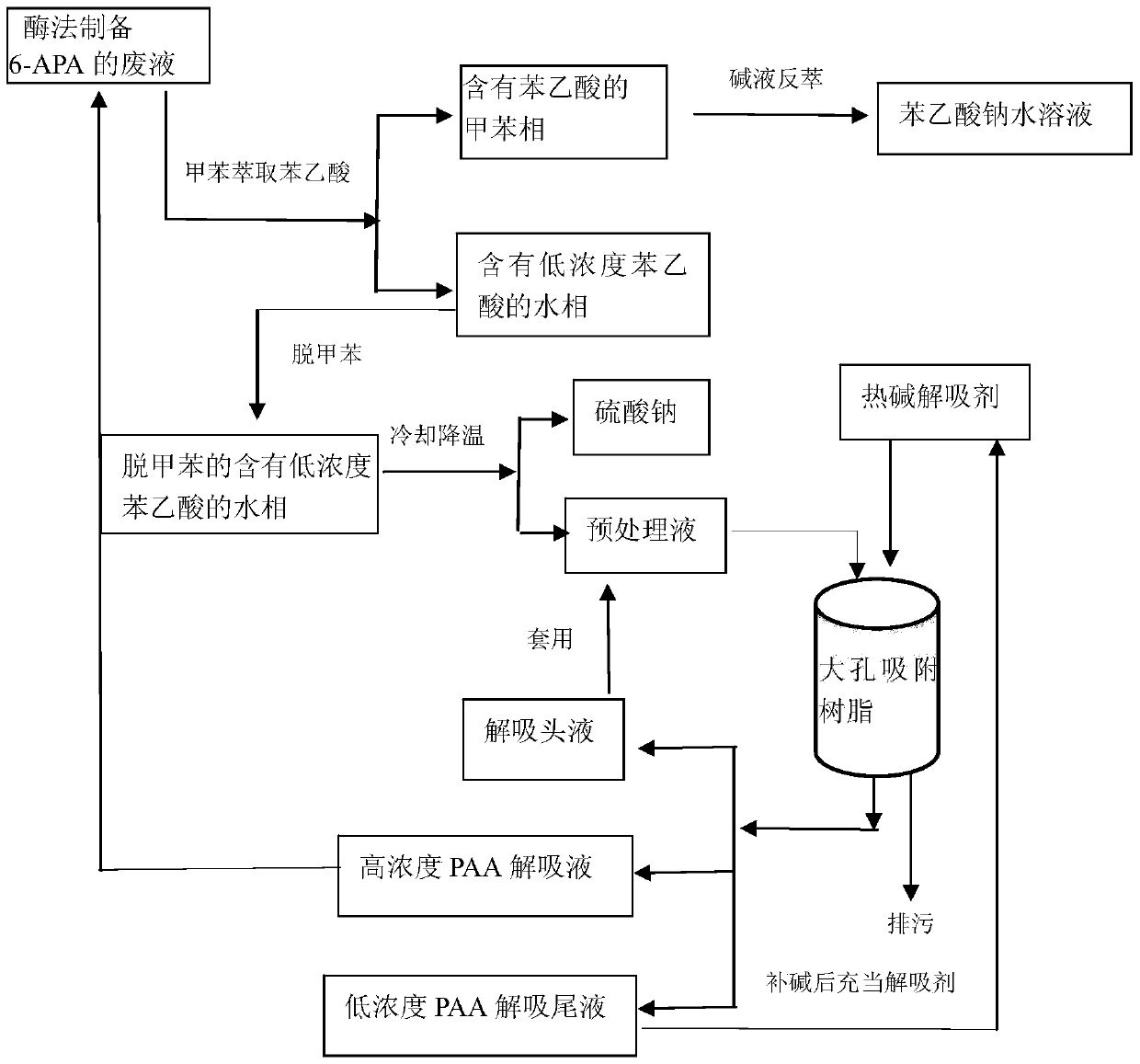
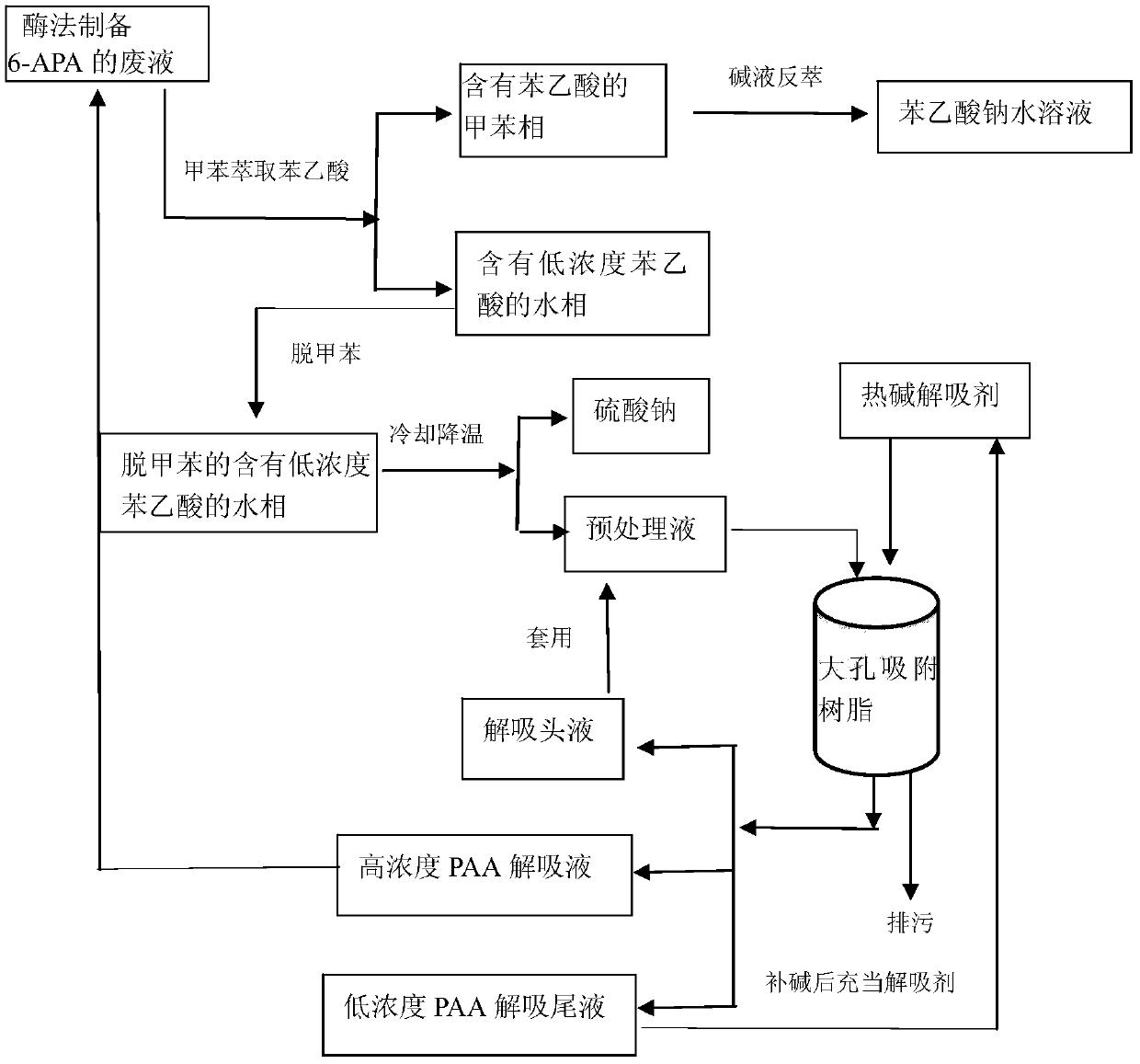
Method for recovering phenylacetic acid from waste liquid of enzymatically preparing 6-aminopenicillanic acid
An aminopenicillanic acid and enzymatic preparation technology, applied in the preparation of carboxylate, chemical instruments and methods, preparation of organic compounds, etc., can solve water and air pollution, difficult procurement, environmental hazards of phenylacetic acid, etc. problems, to achieve the effect of good product quality, easy promotion and application, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Step 1: Extraction of phenylacetic acid with toluene
[0041] Get 400ml of enzymatically prepared waste liquid of 6-APA (light transmittance is 20%, appearance red-black, purity 67%), wherein phenylacetic acid content is 203mg / ml, pH value is 8.9, at temperature 35 ℃, add slowly 200ml of toluene; add 60ml of concentrated sulfuric acid to it to adjust the pH value to 1.95, control the temperature at 68°C, after the interface is clear, let it stand for stratification, that is, the toluene phase containing phenylacetic acid and the water phase containing low concentration of phenylacetic acid; separation 260 ml of toluene phase containing phenylacetic acid and 360 ml of water phase containing low concentration of phenylacetic acid (5.23 mg / ml of phenylacetic acid content) were obtained.
[0042] Step 2: preparing the sodium phenylacetate aqueous solution with few impurities
[0043] Wash 260ml of the toluene phase containing phenylacetic acid obtained in the above step 1 ...
Embodiment 2
[0053] Step 1: Extraction of phenylacetic acid with toluene
[0054] Get 400ml of the waste liquid of 6-APA prepared by enzymatic method (the light transmittance is 22%, the appearance is red and black, the purity is 69%), wherein the content of phenylacetic acid is 195mg / ml, the pH value is 8.9, at a temperature of 37°C, slowly add 200ml of toluene; then add 60ml of concentrated sulfuric acid to adjust the pH value to 1.99, and control the temperature at 68°C. After the interface is clear, let it stand for stratification, that is, the toluene phase containing phenylacetic acid and the water phase containing low-concentration phenylacetic acid; separate 260 ml of toluene phase containing phenylacetic acid and 360 ml of water phase containing low concentration of phenylacetic acid (5.67 mg / ml of phenylacetic acid content) were obtained.
[0055] Step 2: preparing the sodium phenylacetate aqueous solution with few impurities
[0056] Wash 260ml of the toluene phase containing p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| transmittivity | aaaaa | aaaaa |
| transmittivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com