Preparation method of adipic acid dihydrazide
A technology of adipic dihydrazide and adipic acid, which is applied in the field of chemistry, can solve the problems of low yield and achieve the effect of improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
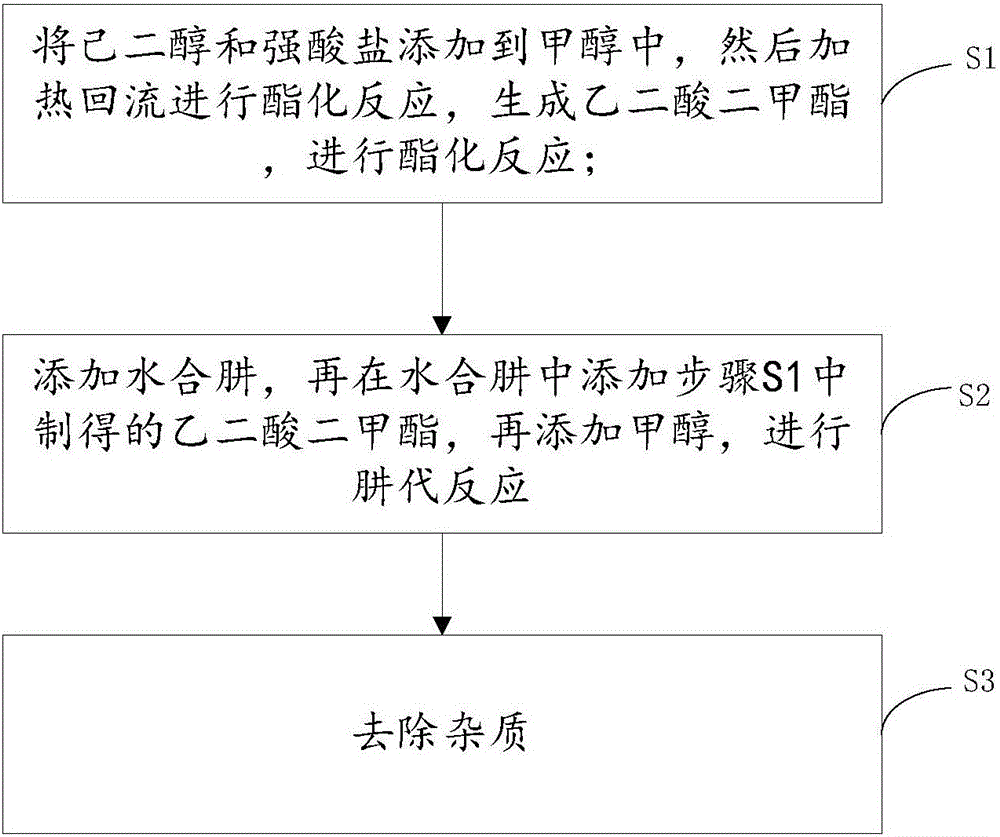
[0028] Such as figure 1 Shown, this adipate dihydrazide preparation method is characterized in that, comprises the steps:
[0029] S1: Add hexanediol and strong acid salt to methanol, then heat to reflux for esterification to generate dimethyl oxalate;
[0030] S2: adding hydrazine hydrate, then adding the dimethyl oxalate prepared in step S1 to the hydrazine hydrate, and then adding methanol to carry out hydrazine substitution reaction;
[0031] S3: Remove impurities.
[0032] The temperature of the esterification reaction in step S1 is 40° C.-80° C., and the temperature is raised for 1-3 hours during reflux.
[0033] During the hydrazino reaction in step S2, the temperature is controlled at 10°C-50°C.
[0034] Removing impurities in step S3 includes removing methanol by distillation and removing catalyst by desalting.
[0035] In step S2, the dimethyl oxalate prepared in step S1 is added to hydrazine hydrate, the temperature is controlled at 40°C-70°C, and the time is 2-...
Embodiment 1
[0042] S1: Add hexanediol and strong acid salt to methanol, and then heat to reflux for esterification to generate dimethyl oxalate for esterification; S2: Add hydrazine hydrate, then add the hydrazine hydrate in step S1 Add methanol to the obtained dimethyl oxalate for hydrazinolation; S3: remove impurities. The temperature of the esterification reaction in step S1 was 40° C., and the temperature was raised for 1 h during reflux. During the hydrazino reaction in step S2, the temperature was controlled at 10°C. Removing impurities in step S3 includes removing methanol by distillation and removing catalyst by desalting. In step S2, add the dimethyl oxalate prepared in step S1 to hydrazine hydrate, and control the temperature at 40° C. for 2 hours. The molar ratio of adipic acid and methanol in the esterification reaction in step S1 is 1:8. The absorption rate of dimethyl oxalate in step S2 is greater than 75%. The molar ratio of adipic acid to dimethyl adipate produced in s...
Embodiment 2
[0044] S1: Add hexanediol and strong acid salt to methanol, and then heat to reflux for esterification to generate dimethyl oxalate for esterification; S2: Add hydrazine hydrate, then add the hydrazine hydrate in step S1 Add methanol to the obtained dimethyl oxalate for hydrazinolation; S3: remove impurities. The temperature of the esterification reaction in step S1 was 80° C., and the temperature was raised for 3 hours at reflux. During the hydrazino reaction in step S2, the temperature is controlled at 50°C. Removing impurities in step S3 includes removing methanol by distillation and removing catalyst by desalting. In step S2, the dimethyl oxalate prepared in step S1 was added to hydrazine hydrate, and the temperature was controlled at 70° C. for 4 hours. The molar ratio of adipic acid and methanol in the esterification reaction in step S1 is 1:10. The absorption rate of dimethyl oxalate in step S2 is greater than 75%. The molar ratio of adipic acid to dimethyl adipate ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com