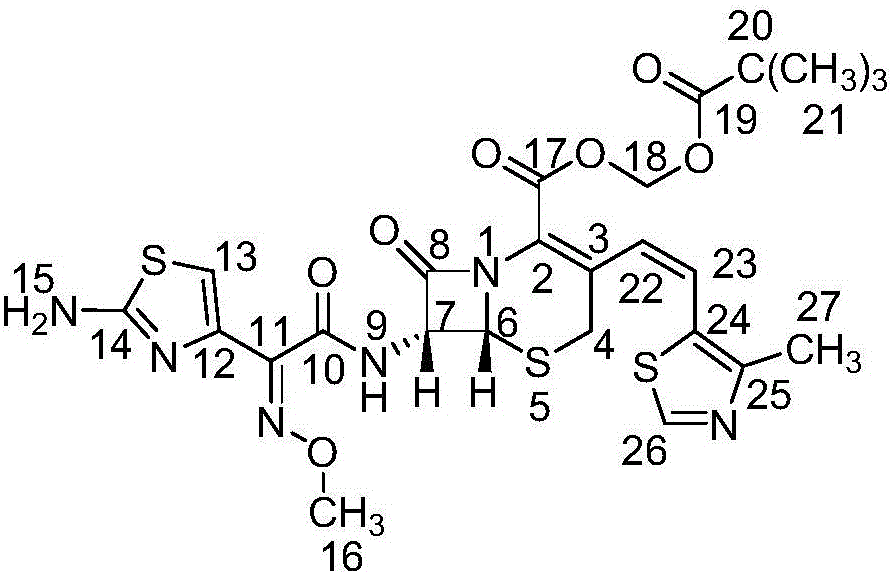
Refinement method of cefditoren pivoxil
A technology of cefditoren pivoxil and a refining method, which is applied in the field of medicine, can solve the problems of high impurity content of cefditoren pivoxil E isomer, low purity of cefditoren pivoxil crude product, non-compliance with USP API standards, etc. , to achieve the effect of simple operation and high refining efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
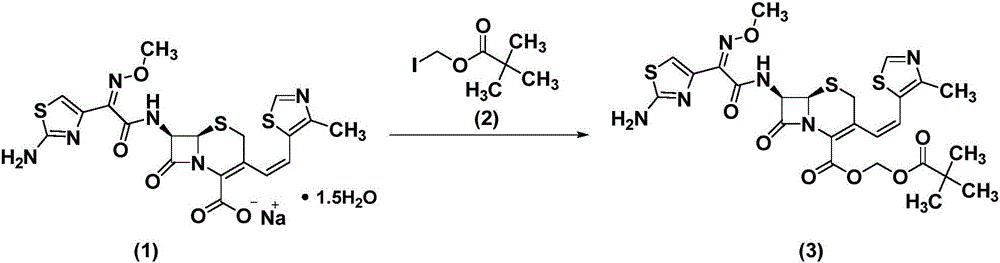
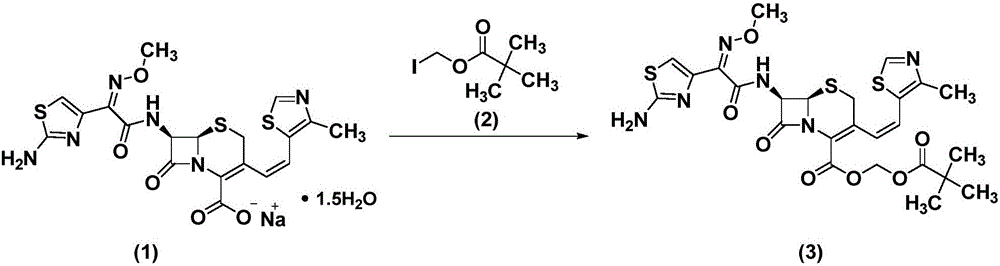
[0029] (1) Synthesis of crude cefditoren pivoxil
[0030] Dissolve 100g compound (1) in 1L dimethyl sulfoxide, lower the temperature to 0°C, add compound (2) dropwise, control the temperature for 1 hour, add 500mL ethyl acetate and 300mL purified water, separate the organic phase, Concentrate and crystallize at ℃ to obtain crude compound (3).
[0031] (2) Refining of crude cefditoren pivoxil
[0032] Dissolve the crude cefditoren pivoxil in 4V / W dimethyl sulfoxide, add ethyl acetate-purified water, extract, concentrate the ethyl acetate phase under reduced pressure at 40°C to 1 / 4V, stir and crystallize 3 Hours; suction filtration, add ethanol to the filter cake, stir at 60°C for 30 minutes, suction filtration, and dry under reduced pressure to obtain a light yellow powder, namely cefditoren pivoxil finished product, purity: 99.2% (plus the main component of the correction factor Self-control method). The proton nuclear magnetic resonance spectrum of cefditoren pivoxil is shown in ...
Embodiment 2
[0037] (1) Synthesis of crude cefditoren pivoxil
[0038] Dissolve 500g of compound (1) in 2L of N,N-dimethylformamide, lower the temperature to -10°C, add compound (2) dropwise, control the temperature and react for 2 hours, add 1L ethyl acetate and 1L purified water, and separate , The organic phase was concentrated and crystallized at 50°C to obtain crude compound (3).
[0039] (2) Refining of crude cefditoren pivoxil
[0040] Dissolve the crude cefditoren pivoxil in 4V / WN,N-dimethylformamide, add ethyl acetate-purified water, extract, and concentrate the ethyl acetate phase at 30°C under reduced pressure to 1 / 4V, and stir Crystallize for 2 hours; filter with suction, add ethanol to the filter cake, stir at 60°C for 20 minutes, filter with suction, and dry under reduced pressure to obtain a light yellow powder, namely the finished product of cefditoren pivoxil, purity: 98.2% (plus correction factor Principal component self-control method).
Embodiment 3
[0042] (1) The synthesis of crude cefditoren pivoxil is the same as in Example 1.
[0043] (2) Refining of crude cefditoren pivoxil
[0044] The crude cefditoren pivoxil of formula (3) was completely dissolved in 4V / WN,N-dimethylformamide, ethyl acetate-purified water was added, extraction, and the ethyl acetate phase was concentrated under reduced pressure at 40°C to 1 / 2V, stirring and crystallization for 2 hours; suction filtration, adding ethanol to the filter cake, stirring at room temperature for 30 minutes, suction filtration, and drying under reduced pressure to obtain a pale yellow powder, the finished product of cefditoren pivoxil, purity: 97.3% ( Principal component self-control method with correction factor).
PUM
| Property | Measurement | Unit |
|---|---|---|
| crystallization temperature | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com