Recombinant protein A as well as coding gene and application thereof
A recombinant protein, coding gene technology, applied in the application, genetic engineering, plant genetic improvement and other directions, can solve the problems of antibody variability, residual impurities, and high ligand shedding rate, to improve adsorption performance, improve tolerance, The effect of prolonged tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] The optimization of embodiment 1 protein A gene
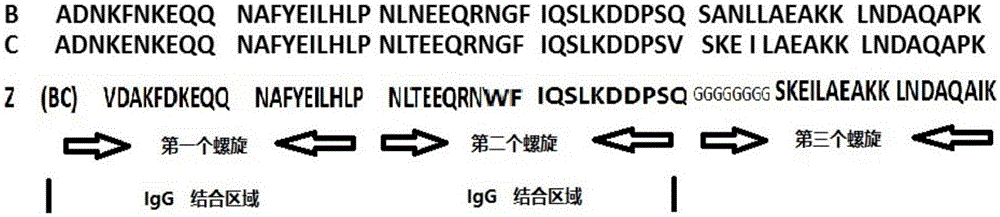
[0018] Such as figure 1 As shown, based on the analysis of the amino acid structure of natural protein A, the amino acid structure of natural protein A can be divided into five highly homologous amino acid regions, namely E, D, A, B, and C, and each region has about 50 amino acids , especially the B domain, which has the highest homology. Many reports believe that the B domain is the key region for antibody binding. Many modifications are based on the B domain, but our modification and transformation, first, the two-amino acid helical structure is based on the B structure At the same time, 8 glycine sequences are added at the back, which are connected to the third helical domain as a connecting segment, which reduces the direct steric hindrance of the three helices and improves the adsorption performance. The third helix takes the C domain and modifies it.
[0019] In the amino acid sequence of protein A, each domain i...
Embodiment 2
[0022] The expression of embodiment 2 recombinant protein A
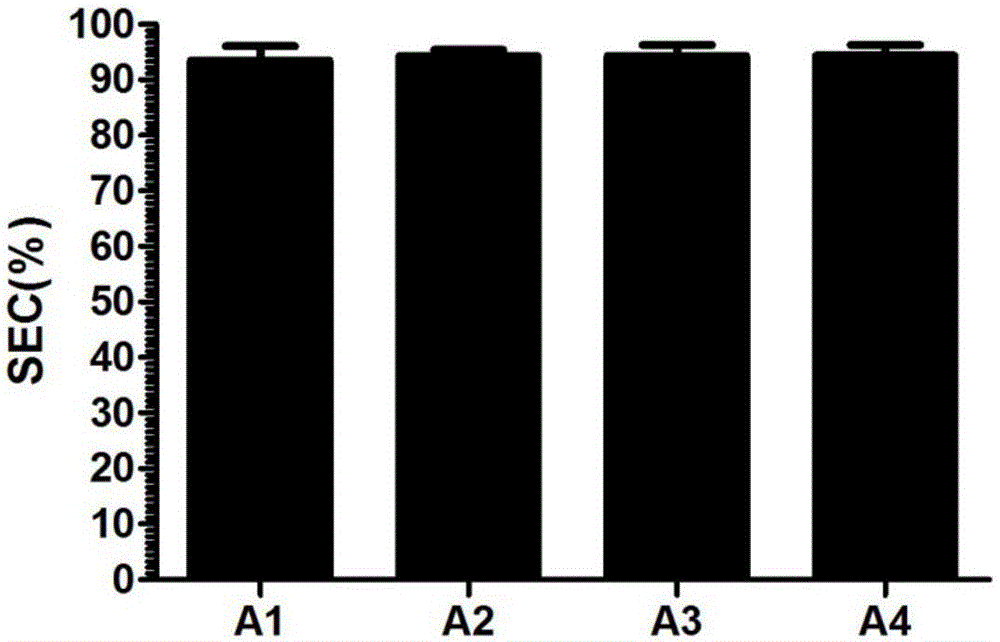
[0023] The above-mentioned synthesized genes were respectively constructed into vectors, cultivated, expressed and purified to obtain recombinant protein A1 solutions, recombinant protein A2 solutions, recombinant protein A3 solutions, and recombinant protein A4 solutions whose sequences corresponded to SEQ ID NO: 1-4.
[0024] The expression vector is PET23a, and a single recombinant plasmid is transformed into Escherichia coli RosettaBlue (DE3) pLysS for fermentation and expression in LB liquid medium. After fermentation, the bacteria are collected and the cell wall is destroyed by thermal cracking to release the expression product out and centrifuge.
[0025] Purify the separation liquid through the IgG affinity medium, load the separation liquid through the affinity medium, wash the medium with 10mM phosphate buffer solution, collect the target protein with a buffer solution of pH 3.8, and adjust it to a neutral...
Embodiment 3
[0026] Example 3 Recombinant Protein A Preparation of Antibody Drug Purification Filler
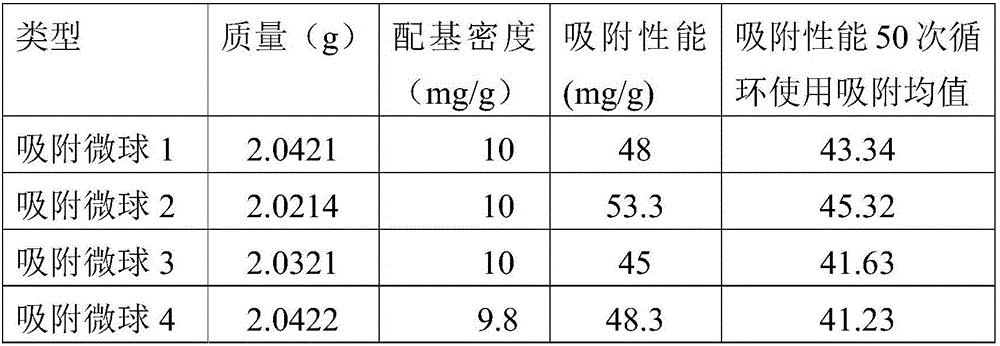
[0027] Recombinant protein A adsorption microspheres 1 to 4 were respectively prepared by using the recombinant proteins A1 to A4 expressed in Example 2, and the preparation process was as follows:
[0028] 1) Microsphere activation
[0029] Bio-sep-6FF is cleaned with purified water, drained in a sand core funnel, weighed 10g of microspheres, 20ml of purified water, 0.2g of sodium hydroxide, 10ml of epichlorohydrin, reacted in a 100ml Erlenmeyer flask, 30°C , 120rpm, reacted in a constant temperature oscillator for 5h, and completed the modification and activation of epichlorohydrin.
[0030] 2) Coupling
[0031] In a 100ml Erlenmeyer flask, add 80ml of recombinant protein A solution (10mg / ml), add activated microspheres 6FF10g, and then add Na 2 CO 3 10mg, KCL 200mg, (NH 4 ) 2 SO 4 500mg, NaOH 400mg, in a constant temperature oscillator, 37°C, 180rpm, 8h, then collect the micro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com