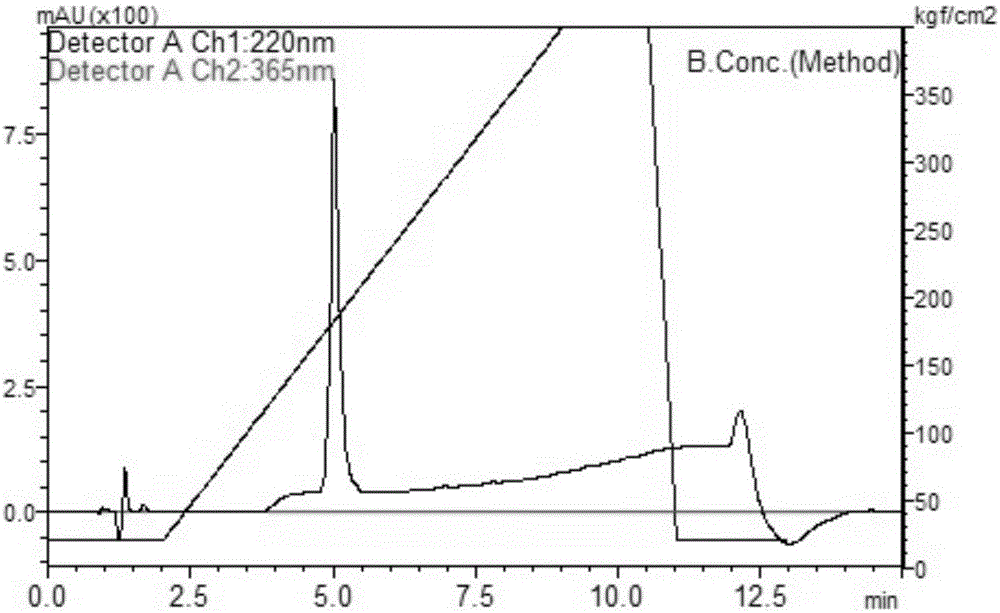
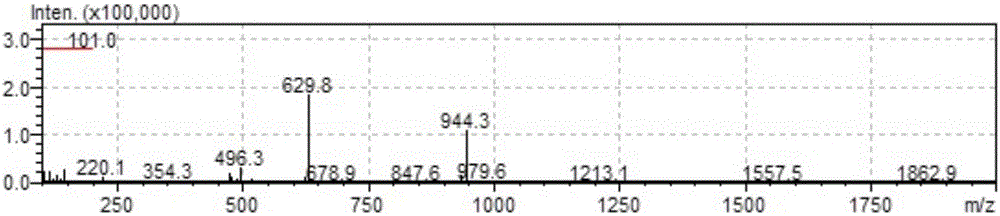
Anti-GRPR antibody, method for producing same, detection method, use of the antibody, kit and gene construct
A gastrin-releasing peptide and antibody technology, applied in the fields of medicine, immunology and molecular biology, can solve the problem of not discovering the invention and making suggestions or expectations, and achieve the effect of auxiliary diagnosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0129] Example 1. Development and acquisition of antigenic peptides
[0130] Most immunogenic sequences of the gastrin releasing peptide receptor were identified using an antigenic index. It is an algorithm commonly used to create linear surface profiles of proteins. It is mainly obtained by computer programs to generate sets of values and combine these sets of values with values predicted from the flexibility and secondary structure regions of polymer chains. This is because most of the antigenic domains are not only present in the surface contact regions of the protein. Therefore, it can be used to identify the second extracellular loop as the most immunogenic followed by ESTNQTFISCAPYPHSN (Glu-Ser-Thr-Asn-Gln-Thr-Phe-Ile-Ser-Cys-Ala-Pro-Tyr-Pro-His -Ser-Asn), this can be found in Figure 11a and Figure 11b see in. The main difference of the natural peptide of the gastrin releasing peptide receptor is that the number of amino acids of the natural peptide is re...
example 2
[0138] Example 2. Development and acquisition of antibodies .
[0139] Synthetic peptides were generated following the sequence ESTNQTFISCAPYPHSN and carried polyclonal antibodies developed by rabbit immunization.
[0140] On the first day, rabbits were inoculated subcutaneously with 200 ug of antigen emulsified into an equal volume of oil emulsion. Two more inoculations with the above-mentioned antigen emulsified into an oil emulsion were carried out at ten-day intervals. Five days after the last vaccination, plasma was collected and tested for specific antibodies to the corresponding antigens using dot blot.
[0141] dot blot
[0142] For immunodetection and quantification of antibodies by dot blot, proteins (approximately 0.5 ug) were added to 0.45 micron nitrocellulose membranes. After drying at room temperature for 30 minutes, the nitrocellulose membrane was blocked with a blocking agent for 1 hour and then incubated with serially diluted serum diluted to 5% for blot...
example 3
[0143] Example 3. Development of reagents and characterization and (or) determination of dysplasia and (or) cervical neoplastic lesions-immunohistochemistry method of quantitative detection.
[0144] After obtaining rabbit polyclonal antibodies, laboratory testing is achieved using immunohistochemical techniques. Paraffin blocks of cervical tissue and tumor and non-tumor tissues of the cervix were selected for immunohistochemical detection. All samples were detected with the antibody of the present invention and a polyclonal anti-rabbit gastrin-releasing peptide receptor control antibody (Affinity BioReagents, Golden, CO, USA, Cat. No.: OPA1-15619) for experimental use only.
[0145] Briefly, after deparaffinization, inactivation of endogenous peroxidases, and cross-reaction with conventional blocking serum, 4 μM sections and diluted primary antibodies (1:200 antibodies in the present invention and 1: 50 control antibody) were incubated overnight at 4°C. Localization of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com