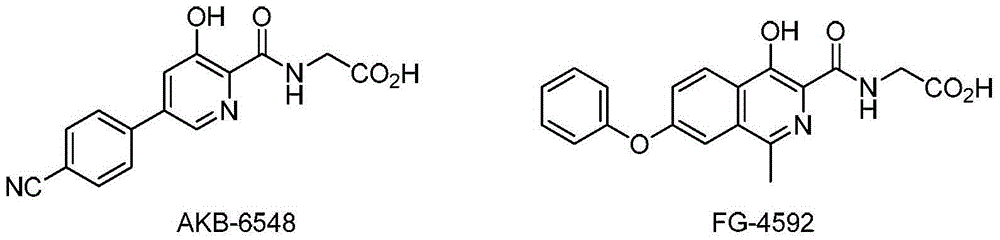
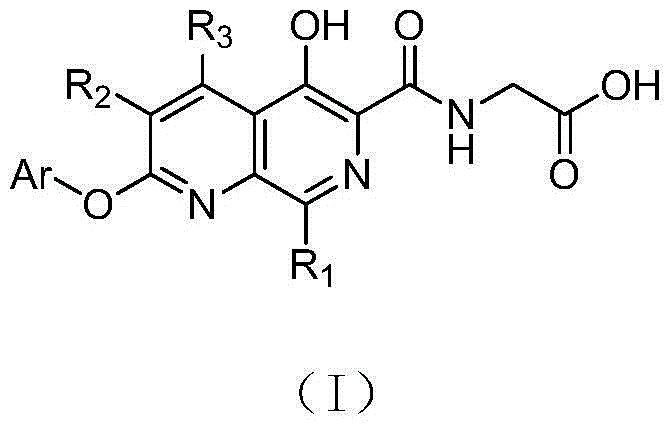
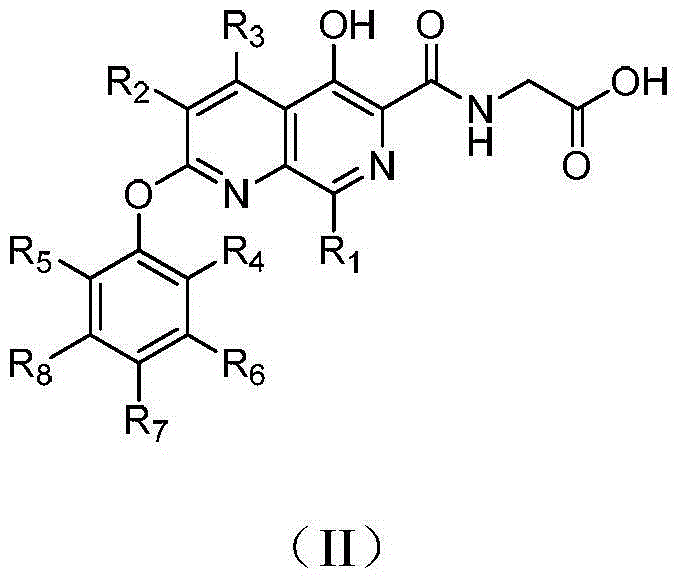
5-hydroxy-1,7-naphthyridine compound substituted by aryloxy group or hetero-aryloxy group, preparation method and pharmaceutical application thereof
A compound and hydroxyl technology, applied in the field of medicine, can solve problems such as short half-life, low bioavailability, and restrictions on patients' self-medication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055]
[0056] Compound No. 1 (2‐(5‐hydroxy‐8‐methyl‐2‐phenoxy‐1,7‐naphthyridine‐6‐carboxamido)acetic acid)
[0057] Step 1: Preparation of methyl 2‐chloro‐6‐phenoxypyridine‐3‐carboxylate
[0058] Add 8.80 g (42.72 mmol) of methyl 2,6-dichloronicotinate and 4.02 g (42.72 mmol) of phenol in sequence in a 250 mL eggplant-shaped bottle, and dissolve them in 45 mL of N,N-dimethylformamide. While stirring, 7.80 mL (55.54 mmol) of triethylamine was added dropwise, and after the dropwise addition, 720 mg (6.41 mmol) of triethylenediamine was added. The reaction was stirred at room temperature for 4-5 hours, and the solution changed from clear to cloudy. TLC analysis [V (petroleum ether) / V (ethyl acetate)=6 / 1] detects that most of the raw materials have reacted. For post-treatment, 1.30 mL of HOAc (21.36 mmol), 25 mL of isopropanol and 15 mL of ice water were sequentially added, the solution changed from turbid to clear, and stirred at room temperature for 0.5 hours. Then, 40 m...
Embodiment 2
[0070]
[0071] Compound No. 2 (2‐(2‐(4‐fluorophenoxy)‐5‐hydroxy‐8‐methyl‐1,7‐naphthyridine‐6‐formylamino)acetic acid)
[0072] Step 1: Preparation of methyl 2‐chloro‐6‐(4‐fluorophenoxy)nicotinate
[0073] Add 2,6-dichloronicotinate methyl ester (2.06 g, 10 mmol) and 4-fluorophenol (1.12 g, 10 mmol) sequentially into a 250 mL eggplant-shaped bottle, 12 ml N,N-dimethylformamide It was dissolved, and triethylamine (1.80ml, 13mmol) was added dropwise under stirring at room temperature. After the dropwise addition, triethylenediamine (168mg, 1.50mmol) was added. The reaction was stirred at room temperature for 4-5 hours, and the solution changed from clear to cloudy. TLC analysis [V (petroleum ether) / V (ethyl acetate)=6 / 1] detects that most of the raw materials have reacted. After treatment, 1.30 mL of HOAc, 25 mL of isopropanol and 15 mL of ice water were added in sequence, the solution changed from turbid to clear, and stirred at room temperature for 0.5 hours. Then, 40 mL...
Embodiment 3
[0086]
[0087] Compound No. 7 (2‐(2‐(4‐chlorophenoxy)‐5‐hydroxy‐8‐methyl‐1,7‐naphthyridine‐6‐formylamino)acetic acid)
[0088] Step 1: Preparation of methyl 2‐chloro‐6‐(4‐chlorophenoxy)nicotinate
[0089] Add 2,6-dichloronicotinate methyl ester (2.06 g, 10 mmol) and 4-chlorophenol (1.57 g, 10 mmol) sequentially into a 250 mL eggplant-shaped bottle, 12 ml N,N-dimethylformamide It was dissolved, and triethylamine (1.80ml, 13mmol) was added dropwise under stirring at room temperature. After the dropwise addition, triethylenediamine (168mg, 1.50mmol) was added. The reaction was stirred at room temperature for 4-5 hours, and the solution changed from clear to cloudy. TLC analysis [V (petroleum ether) / V (ethyl acetate)=6 / 1] detects that most of the raw materials have reacted. After treatment, 1.30 mL of HOAc, 25 mL of isopropanol and 15 mL of ice water were added in sequence, the solution changed from turbid to clear, and stirred at room temperature for 0.5 hours. Then, 40 mL...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com