Hesperetin complex with high oral bioavailability and antioxidant activity and its preparation method and application
An antioxidant activity, hesperetin technology, applied in the direction of organic active ingredients, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc. It can increase the oral bioavailability, enhance the water solubility and prolong the oral bioavailability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
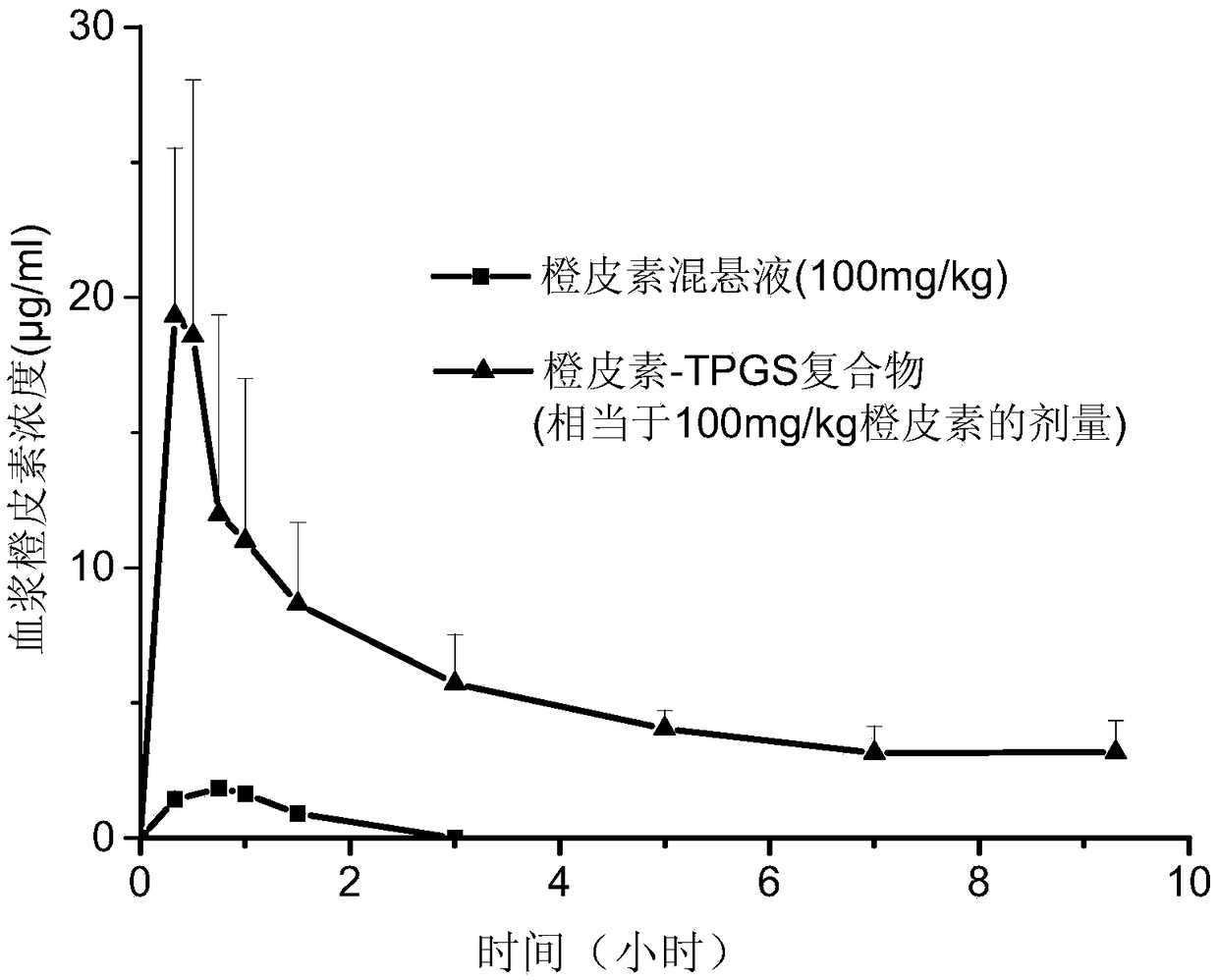
Image
Examples
Embodiment 1
[0042] Raw materials: hesperetin 10mg, TPGS 60mg.
[0043] Preparation process: Dissolve 10mg of hesperetin and 60mg of TPGS in 10ml of ethanol, ultrasonically promote their complete dissolution, 40°C water bath, magnetic stirring for 30min, evaporate under reduced pressure until the solvent is completely volatilized, then freeze-dry to obtain hesperetin and TPGS Hesperetin complex with a mass ratio of 1:6.
[0044] Solubility of the hesperetin complex: in 10ml of water, 10mg of hesperetin dissolves 19.77%.
[0045] The average particle size of the hesperetin complex in water is 32.29nm.
Embodiment 2
[0047] Raw materials: Hesperetin 10mg, TPGS 90mg.
[0048] Preparation process: Dissolve 10mg hesperetin and 90mg TPGS in 10ml ethanol, ultrasonically promote their complete dissolution, 40°C water bath, magnetic stirring for 30min, evaporate under reduced pressure until the solvent is completely volatilized, then freeze-dry to obtain hesperetin and TPGS Hesperetin complex with a mass ratio of 1:9.
[0049] Solubility of the hesperetin complex: In 10ml of water, 10mg of hesperetin dissolves 71.72%.
[0050]The average particle diameter of the hesperetin complex in water is 28.91 nm.
Embodiment 3
[0052] Raw materials: Hesperetin 10mg, TPGS 120mg.
[0053] Preparation process: Dissolve 10mg hesperetin and 120mg TPGS in 10ml ethanol, ultrasonically promote their complete dissolution, 40°C water bath, magnetic stirring treatment for 30min, evaporate under reduced pressure until the solvent is completely volatilized, then freeze-dry to obtain hesperetin and TPGS Hesperetin complex with a mass ratio of 1:12.
[0054] Solubility of the hesperetin complex: In 10 ml of water, 10 mg of hesperetin dissolves 100%.
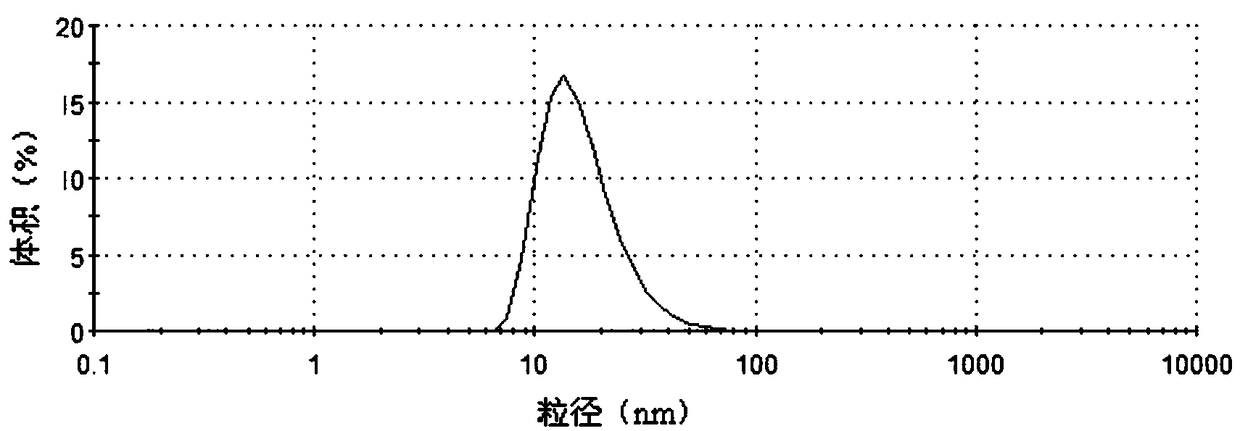
[0055] The average particle diameter of this hesperetin complex in water is 26.15nm, and its particle size distribution figure in water is as follows figure 1 shown.
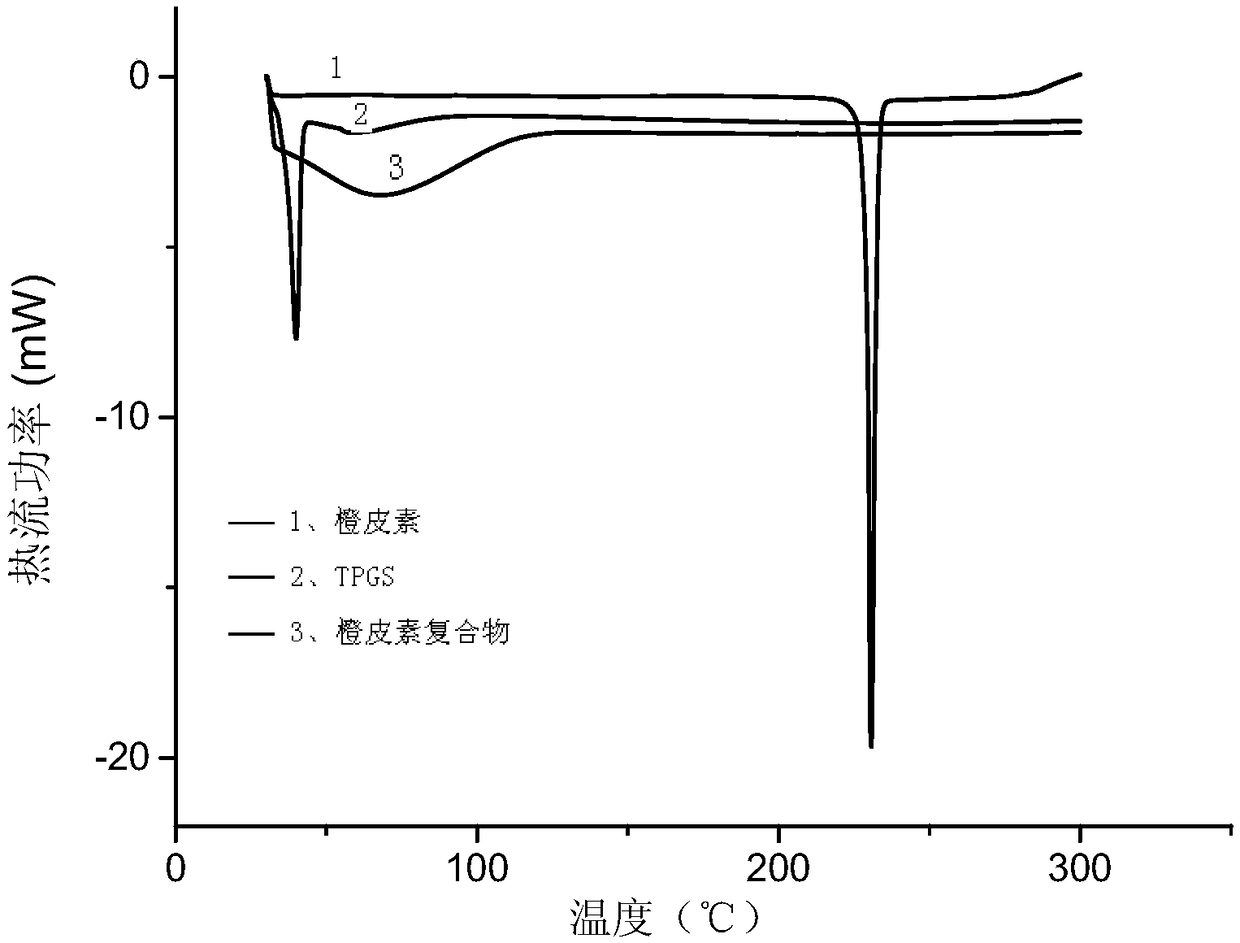
[0056] The hesperetin complex was subjected to differential scanning calorimetry, as figure 2 As shown, the curve of TPGS has a sharp exothermic peak at 40°C, which is the melting point peak, and hesperetin has a sharp exothermic peak at 230°C, which is the melting point peak. After the complex is f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com