Preparation process of 1-(3-chloropyridine-2-yl)-3-bromo-1H-pyrazole-5-formic acid
A preparation process, the technology of chloropyridine, which is applied in the direction of organic chemistry, can solve the problems of complex operation, difficult industrialization, harsh conditions, etc., and achieve the effect of accelerating the reaction, improving the purity, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
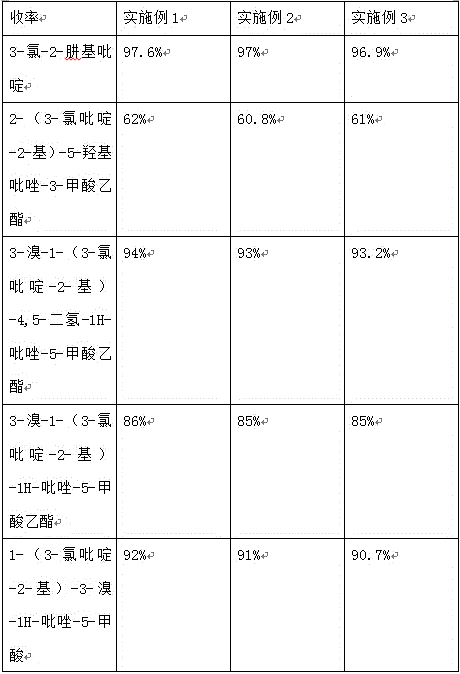
Embodiment 1
[0017] The preparation process of 1-(3-chloropyridin-2-yl)-3-bromo-1H-pyrazole-5-carboxylic acid in this embodiment, the specific steps of the preparation process are as follows:
[0018] (1) Add 70g of 2,3-dichloropyridine and 250ml of ethanol with a mass concentration of 95% to the reaction bottle, add dropwise 125ml of hydrazine hydrate with a mass concentration of 50% under vigorous stirring, and heat to reflux for 25 hours after the addition is completed. After cooling to room temperature, a large amount of white crystals precipitated, filtered and dried to obtain 3-chloro-2-hydrazinopyridine;
[0019] (2) Add 125ml of sodium ethoxide solution into the reaction flask, then add 28g of 3-chloro-2-hydrazinopyridine under reflux, add 42g of diethyl maleate dropwise, drop it for about 1.5h, and continue the reflux reaction for 2.5 h, then lower to room temperature, add 20ml of glacial acetic acid and 300ml of water, after recovering most of the solvent, add 45ml of ethanol wit...
Embodiment 2
[0024] The preparation process of 1-(3-chloropyridin-2-yl)-3-bromo-1H-pyrazole-5-carboxylic acid in this embodiment, the specific steps of the preparation process are as follows:
[0025] (1) Add 60g of 2,3-dichloropyridine and 200ml of ethanol with a mass concentration of 95% in the reaction bottle, add dropwise 100ml of hydrazine hydrate with a mass concentration of 50% under vigorous stirring, and heat to reflux for 20 hours after the addition is completed. After cooling to room temperature, a large amount of white crystals precipitated, filtered and dried to obtain 3-chloro-2-hydrazinopyridine;
[0026] (2) Add 100ml of sodium ethoxide solution into the reaction flask, then add 25g of 3-chloro-2-hydrazinopyridine under reflux, add 40g of diethyl maleate dropwise, drop it for about 1h, and continue the reflux reaction for 2h. Then drop to room temperature, add 15ml of glacial acetic acid and 250ml of water, after recovering most of the solvent, add 40ml of ethanol with a ma...
Embodiment 3
[0031] The preparation process of 1-(3-chloropyridin-2-yl)-3-bromo-1H-pyrazole-5-carboxylic acid in this embodiment, the specific steps of the preparation process are as follows:
[0032](1) Add 80g of 2,3-dichloropyridine and 300ml of ethanol with a mass concentration of 95% in the reaction bottle, add dropwise 150ml of hydrazine hydrate with a mass concentration of 50% under vigorous stirring, and heat to reflux for 30 hours after the addition is completed. After cooling to room temperature, a large amount of white crystals precipitated, filtered and dried to obtain 3-chloro-2-hydrazinopyridine;
[0033] (2) Add 150ml of sodium ethoxide solution into the reaction flask, then add 30g of 3-chloro-2-hydrazinopyridine under reflux, add 45g of diethyl maleate dropwise, drop it for about 2h, and continue the reflux reaction for 3h. Then cool down to room temperature, add 25ml of glacial acetic acid and 350ml of water, after recovering most of the solvent, add 50ml of ethanol with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com