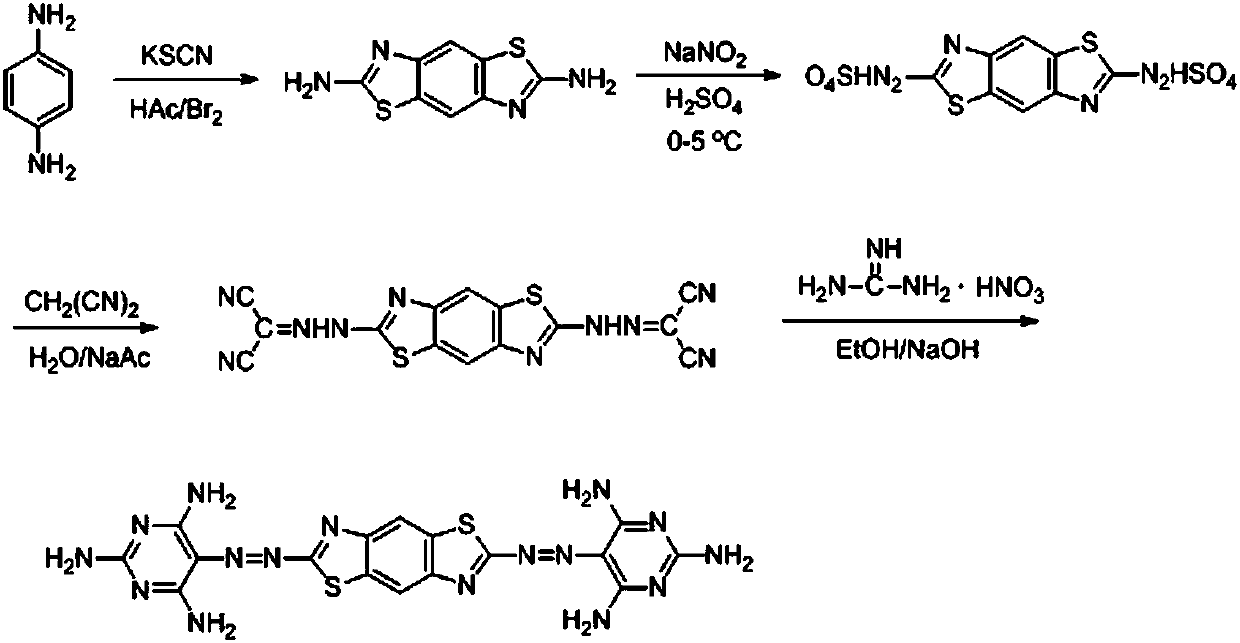
Preparation method and application of 2, 6-bis(2,4, 6-triamino-5-pyrimidinazo)benzo(1, 2-d; 4, 5-d) bithiazole
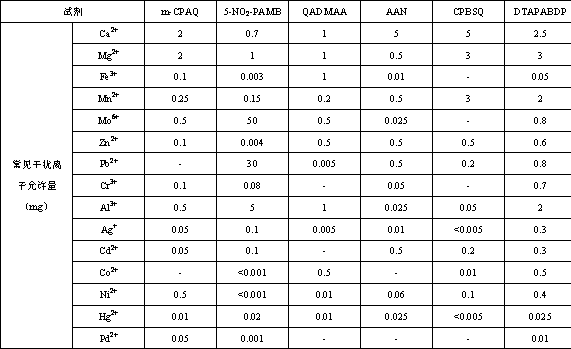
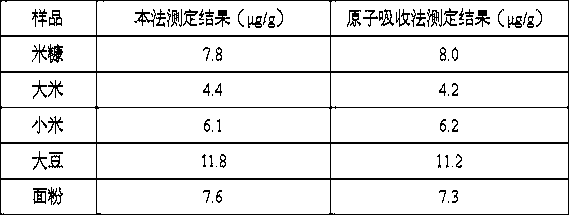
A technology for pyrimidine azo and diaminobenzene, which is applied in the field of disazo compounds, can solve the problems of unsatisfactory sensitivity and selectivity of reagents for detecting heavy metal ions, and achieve the effects of stable test system, high sensitivity and fast response speed.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1. One kind of 2,6-bis(2,4,6-triamino-5-pyrimidylazo)benzo(1,2-d; 4,5-d)bithiazole (DTAPABDP), its molecular The structural formula is:
[0045] .
[0046] The preparation method of 2,6-bis(2,4,6-triamino-5-pyrimidylazo)benzo(1,2-d; 4,5-d)bithiazole is as follows:
[0047] (1) Preparation of 2,6-diaminobenzo(1,2-d; 4,5-d)bithiazole
[0048] Add 10.81 g (0.1 mol) of 4,4´-p-phenylenediamine, 150 mL of glacial acetic acid and 70 g of potassium thiocyanate to a dry 500 mL three-necked flask equipped with a reflux condenser with a drying tube and a dropping funnel. Stir at room temperature for 20min, then measure 10mL of bromine and dissolve it in 130mL of glacial acetic acid, add it to the reaction mixture, keep the reaction temperature at 45-55°C, continue stirring for 24h, pour the mixture into cold water, and add to the mixture under stirring Add ammonia water, adjust the pH to 8-9, let it stand, after the solution is cooled, suction filter, wash with water ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com