Immune library-derived nano-antibody for specifically recognizing immunoglobulin Fc fragment
A technology of immunoglobulin and nanobody, applied in the field of single domain heavy chain antibody, which can solve the problems of high production cost and cumbersome preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Construction of Anti-Fc Single Domain Heavy Chain Antibody Immune Library
[0026] After emulsifying 300 μg of Fc recombinant protein (the protein can be obtained through commercial channels) with complete Freund's adjuvant, multi-point subcutaneous injection was performed to immunize alpacas (Lama pacos). 150 μg Fc recombinant protein was emulsified with Freund's incomplete adjuvant for booster immunization, and the interval was 2 weeks. Blood was collected from vein 7 days after each immunization, and the serum titer was determined by indirect ELISA method. The sample with the highest serum titer was selected to separate lymphocytes. Extract RNA.
[0027] The extraction of RNA was carried out according to the instruction manual of RNAiso reagent from TAKARA company. Using RNA as a template and oligo dT as a primer, the first strand of cDNA was synthesized according to the instructions of TAKARA reverse transcriptase.
[0028] The gene encoding the variable region of...
Embodiment 2
[0036] Panning and Identification of Anti-IgG Fc Fragment Single Domain Heavy Chain Antibody
[0037] A solid-phase affinity panning method was used to pan out a single-domain heavy-chain antibody directed against the IgG Fc segment from an anti-Fc single-domain heavy-chain antibody immune library. The mouse serum was purified by affinity chromatography to obtain an IgG solution. Dilute the IgG solution with PBS to 50-100 μg / mL, add 100 μL to each well, and coat overnight at 4°C; suck out the coating solution, wash the plate with PBS 3 times, add 300 μL 3% BSA-PBS to each well, block for 2 hours at 37°C ; Wash the plate 6 times with PBS, add 100 μL phage antibody library (containing about 2×10 11 CFU), 37°C, incubate for 1.5 h; aspirate unbound phage, wash the plate with PBST (containing 0.5% Tween-20) 5 times (increase 1 time per round), and then wash the plate with PBS 10 times (the number of washes increases gradually 5 rounds); 100 μL of eluent (glycine-hydrochloric acid...
Embodiment 3
[0048] Expression and purification of anti-IgG Fc fragment single domain heavy chain antibody in Escherichia coli.
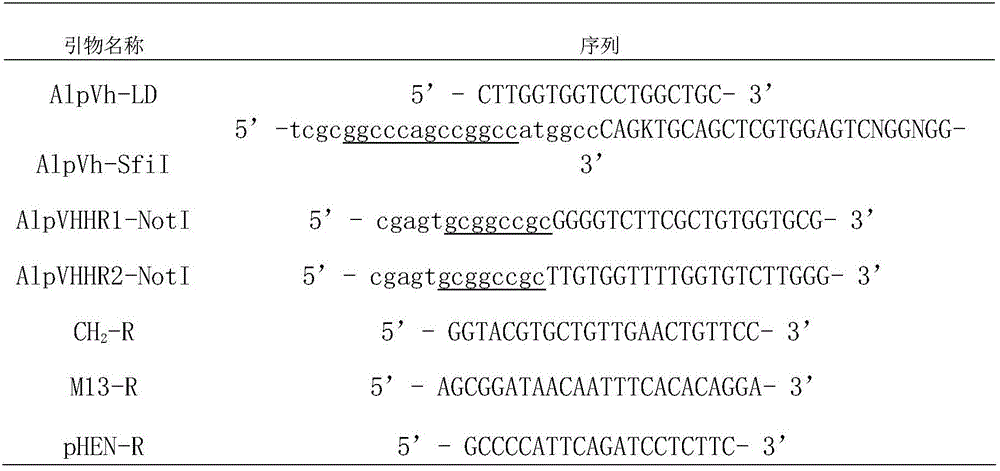
[0049] Acquisition of the DNA fragment encoding the anti-IgG Fc segment single-domain heavy chain antibody: 1. Use restriction endonuclease SfiI / NotI, double-digest the phagemid pHEN-X, and recover the anti-IgG Fc segment single domain by agarose gel electrophoresis Heavy chain antibody gene; 2. Directly send the anti-IgG Fc segment single domain heavy chain antibody coding sequence to a biotechnology service company for chemical synthesis; 3. Design specific primers and use PCR technology from the cDNA library derived from alpaca (Lama pacos) amplified in.
[0050] The obtained anti-IgG Fc-segment single-domain heavy-chain antibody gene fragment was cloned into the expression vector pRXS, identified by PCR and enzyme digestion, and the E. coli expression plasmid for the anti-IgG Fc-segment single-domain heavy-chain antibody was constructed, named pRXS-X.
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com