Method for preparing recombinant bacillus subtilis plasmin and enteric capsule thereof
A technology of Bacillus subtilis and enteric-coated capsules, applied in biochemical equipment and methods, capsule delivery, medical preparations containing active ingredients, etc., can solve the problems of not exerting important medicinal value, high purification cost, complex fermentation products, etc. , to achieve huge development potential and market application value, easy industrialization amplification, and huge market benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
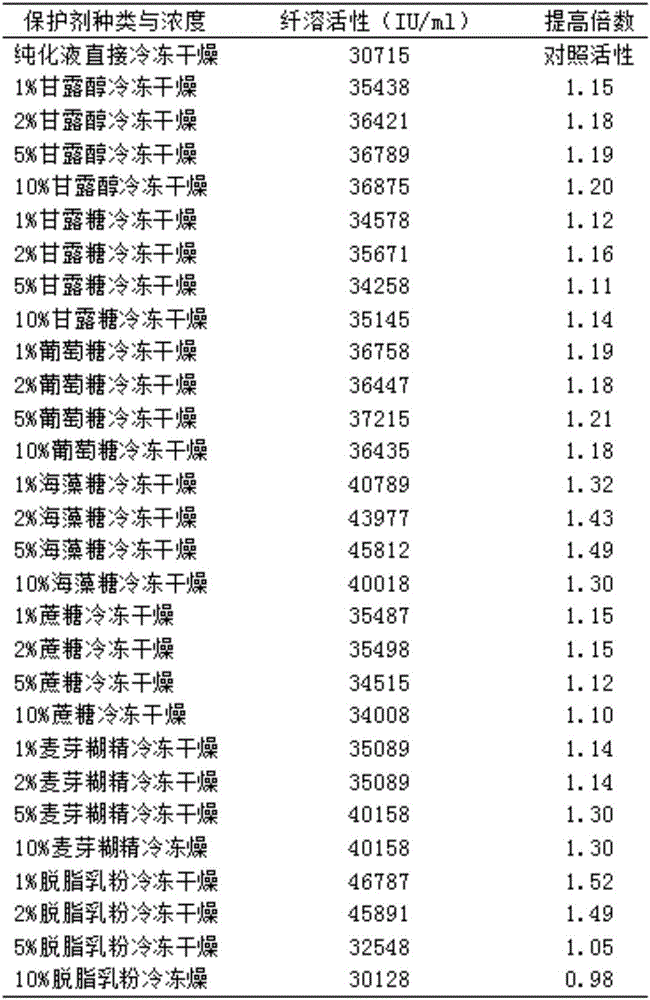
[0046] A method for purifying recombinant Bacillus subtilis plasmin, the method for purifying comprises the following steps:
[0047] 1) Select the filler for protein purification; according to the characteristics of the recombinant Bacillus subtilis plasmin protein, select the filler according to the purification efficiency and final protein purification purity (Table 1). Both Phenyl Sepharose 6FF (HS) and Benzamidine 4FF (HS) have a purity of more than 95%. Considering the cost factor, the final comprehensive comparison selection determines that the purified filler is Phenyl Sepharose 6FF (HS) (phenyl agarose).
[0048] 2) Purifying the yeast fermentation liquid through a hydrophobic chromatography filler to obtain a purified liquid;
[0049] 3) The purified liquid is further processed by desalting chromatography with G25 (dextran G-25) filler to obtain purified recombinant Bacillus subtilis fibrinolytic enzyme.
[0050] The hydrophobic chromatography includes a hydrophobic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com