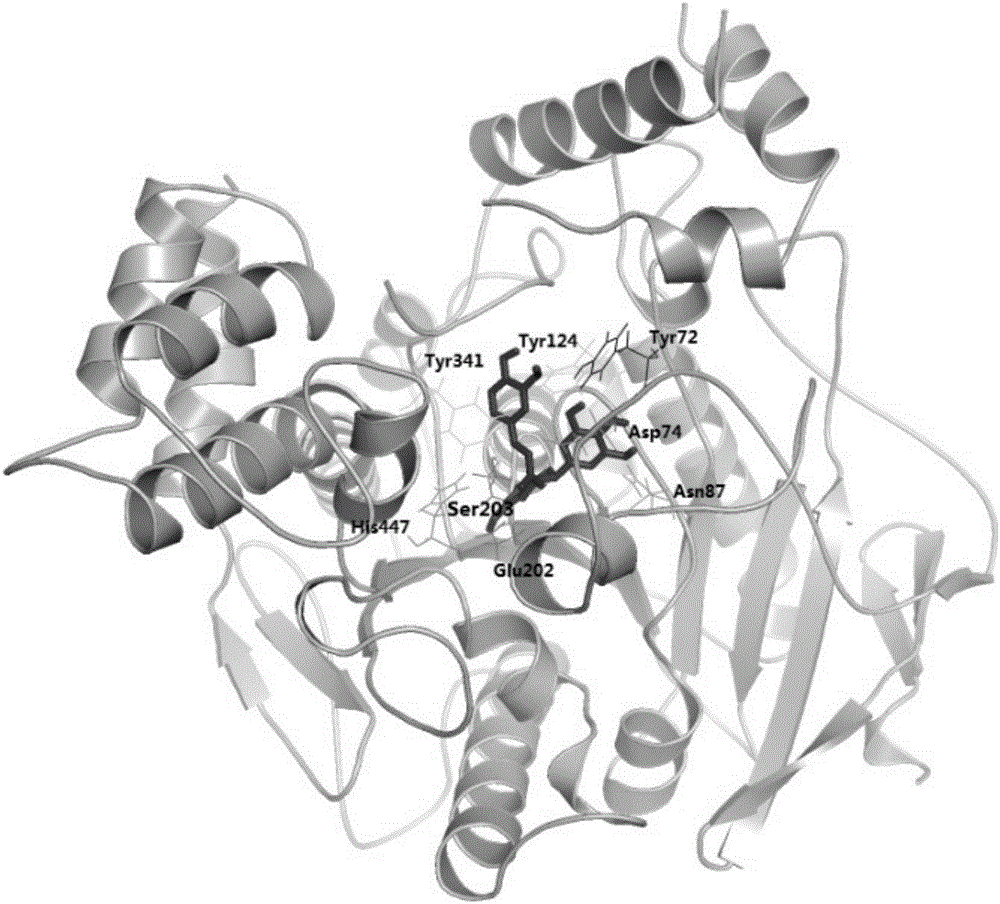Catechin derivative and use thereof in preparation of acetylcholinesterase activity inhibition medicines
A technology of catechin and derivatives, applied in the field of acetylcholinesterase inhibitors, to achieve the effect of inhibiting acetylcholinesterase activity, simple preparation method, and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Preparation of catechin derivatives
[0037] 1.1 Description of catechin derivatives
[0038] The structure of catechin derivatives is shown in formula (I):
[0039]
[0040] When R 1 = H, R 2 When =OH, epicatechin trans-caffeate is formed;
[0041] When R 1 =OH,R 2 When =H, formed epigallocatechin trans p-coumarate;
[0042] When R 1 =OH,R 2 =OCH 3 When, epigallocatechin (3″-methoxy)-trans p-coumarate is formed.
[0043] 1.2 Specific preparation methods and results
[0044] (1) Get 4.5 kilograms of Zijuan tea, and pulverize Zijuan tea;
[0045] (2) First, 4.5 kg of pulverized Zijuan tea was leached with 15 liters of petroleum ether at room temperature for 72 hours, and then ultrasonically leached at 70 Hz for 2 hours to obtain a petroleum ether extract; leaching three times according to the above steps, combined extraction liquid and concentrate the extract to a paste to obtain 18 grams of petroleum ether extract;
[0046] Then the Zijuan tea extracted ...
Embodiment 2
[0077] Inhibitory Effect of Molecular Mimetic Catechin Derivatives on Acetylcholinesterase
[0078] Experimental method and results
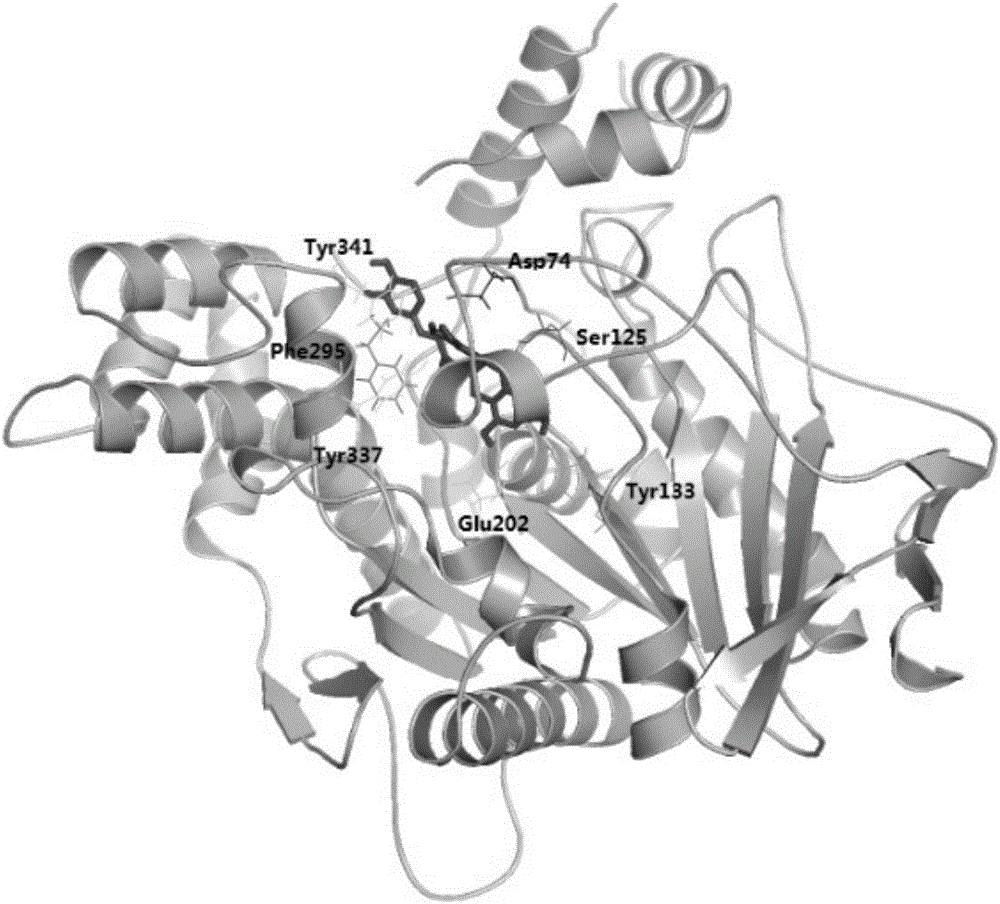
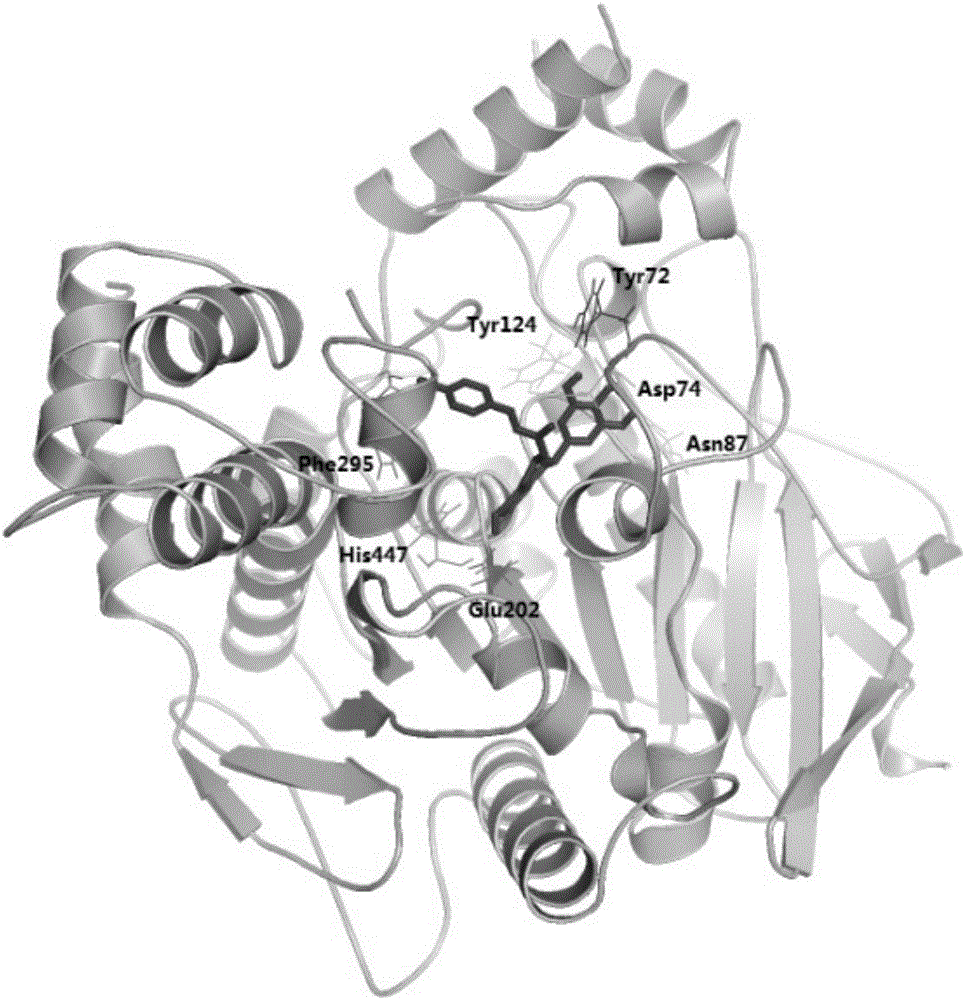
[0079] Molecular docking was used to simulate the molecular binding modes of the above three catechin derivatives to acetylcholinesterase (AchE). The software used for molecular docking is AutoDock 4.2, and the corresponding scores can be obtained after the simulation ( E is the binding energy, Ki is the inhibition constant), the higher the score, the stronger the predicted affinity between the two. The binding amino acid residues found by molecular docking are:
[0080] Epicatechin trans-caffeate: Asp74, Ser125, Tyr133, Glu202, Phe295, Tyr337, Tyr341;
[0081] Epigallocatechin trans-p-coumarate: Tyr72, Asp74, Asn87, Tyr124, Glu202, Phe295, His447;
[0082] Epigallocatechin (3″-methoxy)-trans-p-coumarate: Tyr72, Asp74, Asn87, Tyr124, Glu202, Ser203, Tyr341, His447.
[0083] The docking results are as follows figure 1 , 2 , 3 shown. The ...
Embodiment 3
[0086] Verification of Inhibitory Effect of Catechin Derivatives on Acetylcholinesterase
[0087] 3.1 Experimental materials and reagents
[0088] Huperzine A, PBS buffer (pH 8.0±0.1), acetylcholinesterase (AchE) (0.25U·mL -1 ), thioacetylcholine iodide (ATCI) (0.0025mol L -1 ), 5,5-dithiodinitrobenzoic acid (DTNB) (0.003mol L -1 ), Dimethylsulfoxide (DMSO)
[0089] 3.2 Experimental methods and results
[0090] Add 160 μL of DTNB solution and 50 μL of AchE solution in 96-well plate, add 50, 10, 5, 1, 0.1 μmol·L respectively -1 Add 10 μL of epicatechin trans-caffeate solution, heat at 37°C for 20 minutes, add 30 μL of ATCI (solution, place in a microplate reader (25°C), record the A value of the reaction solution under visible light with a wavelength of 412nm .All samples were repeated 3 times and recorded as sample A. Replace AchE with 50 μL of PBS, replace the sample solution with 10 μL of DMSO, record it as A blank (repeat 3 times), and do blank zero adjustment. Replace...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com