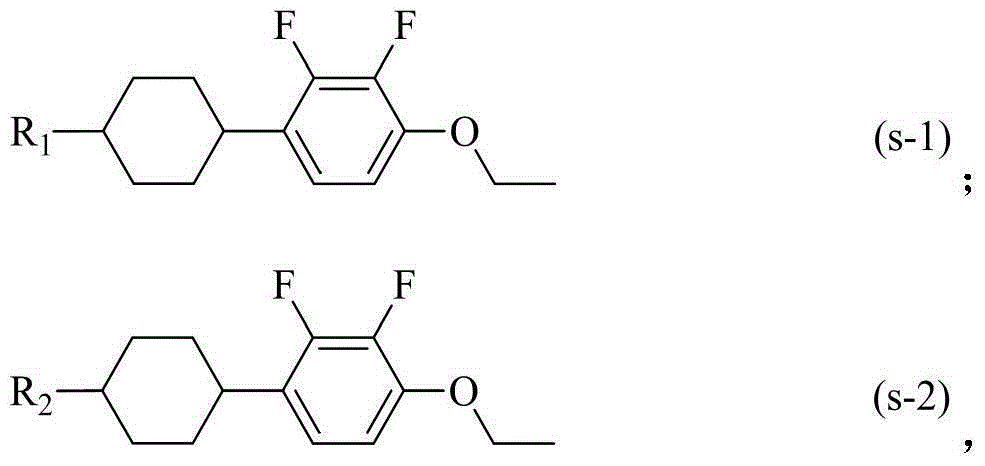
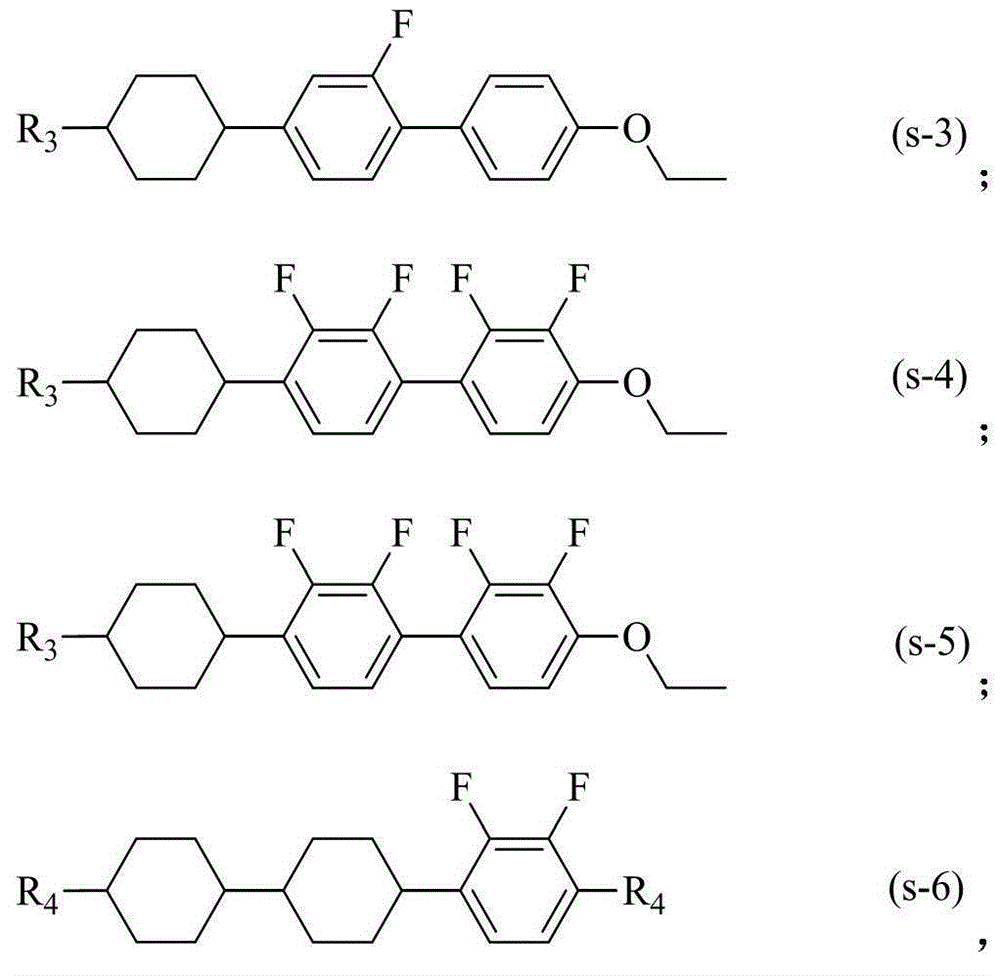
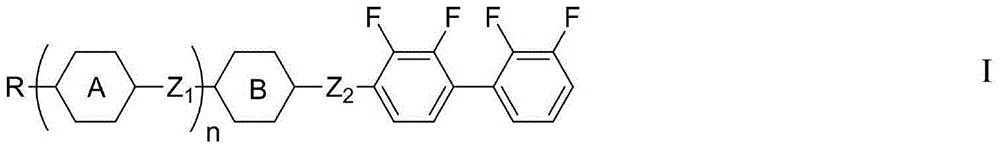
Liquid crystal compound having dielectric anisotropy, composition thereof and application thereof
A liquid crystal compound, anisotropic technology, applied in the liquid crystal field, can solve problems such as high viscosity, insufficient dielectric constant anisotropy, and lower driving voltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0121] The synthetic route of compound 3CWW is as follows:
[0122]
[0123] Compound A and 2,3-difluorobromobenzene were all from Jiangsu Hecheng New Materials Co., Ltd.
[0124] Its specific preparation process is as follows:
[0125] 1) Synthesis of Compound B
[0126] Add 23.8g of compound A and 250mL of anhydrous THF (tetrahydrofuran) into a 1L three-necked flask, and under nitrogen protection, cool down to -78°C, add 42mL of l n-BuLi n-hexane solution (2.4mol / L) dropwise, after the dropwise addition, Stir at -78°C for 1h, then dropwise add 23g of B(i-BuO) 3 and 80mL of anhydrous TFT, after the dropwise addition, keep stirring at -78°C for 1h, then naturally warm up to room temperature, pour the reaction solution into a mixture of 100mL 5% dilute hydrochloric acid and 200g ice, stir, separate the liquid, and diethyl acetate Ester extracted the water layer, combined the oil layers, evaporated the solvent, added 150mL 90-120°C petroleum ether for slurry, filtered to o...
Embodiment 2
[0134] The synthetic route of compound 3C1OWW is as follows:
[0135]
[0136] Propylcyclohexylmethanol p-toluenesulfonate was from Jiangsu Hecheng New Materials Co., Ltd.
[0137] Its specific preparation process is as follows:
[0138] 1) Synthesis of Compound E
[0139] Add 15.8g of compound D, 19.3g of 2,3-difluorobromobenzene, 120mL of toluene, 60mL of ethanol, 60mL of water, 42.4g of sodium carbonate into a 500mL three-necked flask, and add 0.5g of Pd(PPh 3 ) 4 , heated to reflux for 6h, cooled to room temperature, added 100mL of water, separated the layers, extracted the water layer with 100mL of toluene, combined the oil layer, evaporated the solvent, and purified by column chromatography to obtain 15.4g of white solid compound E, yield: 68.1%, GC>98%.
[0140] 2) Synthesis of Compound F
[0141] Add 15.4g of compound E and 200mL of anhydrous THF into a 500mL three-necked flask, and under nitrogen protection, cool down to -78°C, add 30mL of n-BuLi n-hexane solu...
Embodiment 3
[0149] The synthetic route of compound 3CWWW is as follows:
[0150]
[0151] Its specific preparation process is as follows:
[0152] 1) Synthesis of Compound J
[0153] Add 23.8g of compound A and 250mL of anhydrous THF to a 1L three-necked flask, and under nitrogen protection, cool down to -78°C, add 42mL of n-BuLi n-hexane solution (2.4mol / L) dropwise, after the dropwise addition, -78°C Insulated and stirred for 1h, then added dropwise by 25.4g I 2and 150mL of anhydrous TFT, after the dropwise addition, keep stirring at -78°C for 1h, then naturally warm up to room temperature, pour the reaction solution into a mixture of 100mL 5% dilute hydrochloric acid and 200g of ice, stir, separate the liquid, and diethyl acetate Extract the water layer with ester, combine the oil layers, wash the oil layer with sodium thiosulfate aqueous solution, wash the oil layer with water until neutral, evaporate the solvent, and purify by column chromatography to obtain 25.6g of light yello...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com