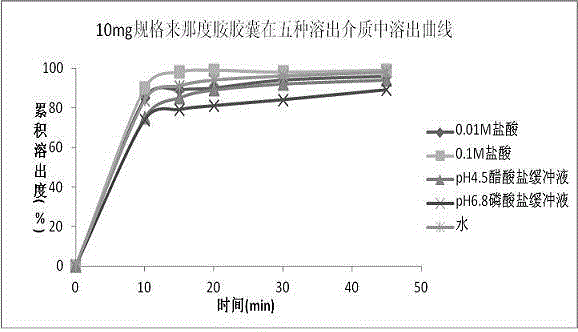
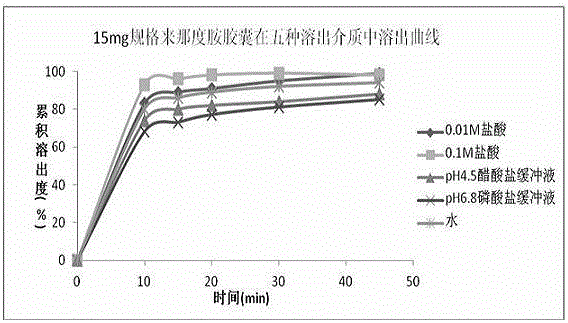
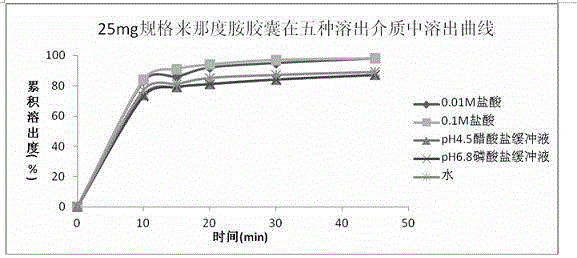
Pharmaceutical composition containing lenalidomide, and preparation method and medical application thereof
A technology of lenalidomide and composition, applied in the field of pharmaceutical preparations, can solve the problems of high production cost, slow drug dissolution, low preparation efficiency and the like, and achieve the effect of quality, safety and controllability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] Composition and dosage of capsule contents containing 5 mg of active ingredient (200 capsules)
[0086] components Dosage Lenalidomide 1.000g anhydrous lactose 29.400g microcrystalline cellulose 8.000g Croscarmellose Sodium 1.200g Magnesium stearate 0.400g
[0087] Preparation Process:
[0088] 1) Pretreatment of raw and auxiliary materials: lenalidomide is micronized and passed through a 60-mesh sieve, and microcrystalline cellulose and croscarmellose sodium are passed through a 60-mesh sieve for treatment.
[0089] 2) Add the prescribed amount of microcrystalline cellulose into the laboratory hopper mixer, set the speed at 15 rpm, and mix for 3 minutes;
[0090] 3) Add the prescription amount of lenalidomide and croscarmellose sodium into the laboratory hopper mixer, add anhydrous lactose in equal increments, set the speed at 15 rpm, and mix for 20 minutes;
[0091] 4) Magnesium stearate is added to the laboratory hopper...
Embodiment 2
[0094] Composition and dosage of capsule contents containing 5 mg of active ingredient (200 capsules)
[0095] components Dosage Lenalidomide 1.000g corn starch 29.400g microcrystalline cellulose 8.000g Croscarmellose Sodium 1.200g Magnesium stearate 0.400g
[0096] The preparation process refers to Example 1.
Embodiment 3
[0098] Composition and dosage of capsules containing 10 mg of active ingredient (200 capsules)
[0099] components Dosage Lenalidomide 2.00g anhydrous lactose 54.80g microcrystalline cellulose 20.00g Crospovidone 2.40g Magnesium stearate 0.8g
[0100] The preparation process refers to Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Angle of repose | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com